手性n -取代2-氨基-2-苯基乙醇形成非对映体盐分离2-卤代苯胺酸对映体。
IF 2.8
4区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
通过非对映体盐的形成,开发了手性n取代的仲1,2氨基醇,用于对邻卤代苯甲酸(o-X-MAs)的对映分离。两种结构异构体n -甲基-2-氨基-1,2-二苯乙醇和n -苄基-2-氨基-2-苯乙醇对o-X-MAs (X = Cl, Br, I)具有较高的分离能力。通过对难溶盐的晶体学分析,阐明了手性识别机制。溶解剂氮原子上的取代基和结晶溶剂的包裹体稳定了盐,提高了盐的分离能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
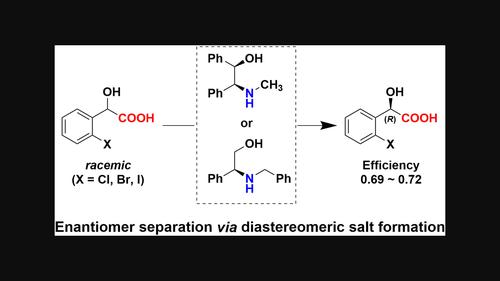
Enantiomer separation of 2-halomandelic acids via diastereomeric salt formation with chiral N-substituted 2-amino-2-phenylethanols
Chiral N-substituted secondary 1,2-aminoalcohols have been developed for the enantioseparation of ortho-halomandelic acids (o-X-MAs) via diastereomeric salt formation. Two structural isomers, N-methyl-2-amino-1,2-diphenylethanol and N-benzyl-2-amino-2-phenylethanol, showed high separation abilities for o-X-MAs (X = Cl, Br, I). The chiral recognition mechanism was elucidated by crystallographic analysis of the less-soluble salts. The substituents on the nitrogen atom of the resolving agents and the inclusion of the crystallization solvent stabilized the salt and enhanced their separation ability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Chirality
医学-分析化学
CiteScore
4.40
自引率
5.00%
发文量
124
审稿时长
1 months
期刊介绍:
The main aim of the journal is to publish original contributions of scientific work on the role of chirality in chemistry and biochemistry in respect to biological, chemical, materials, pharmacological, spectroscopic and physical properties.
Papers on the chemistry (physiochemical, preparative synthetic, and analytical), physics, pharmacology, clinical pharmacology, toxicology, and other biological aspects of chiral molecules will be published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


