合成2-烷基取代四氢喹啉生物碱(−)库柏碱的收敛方法
Q2 Chemistry
引用次数: 2
摘要
通过(R)-苄基- 2-甲酰基-3,4-二氢喹啉-1(2H)-羧酸酯对映特异性构造合成2-烷基取代四氢喹啉生物碱(−)-库柏碱的收敛方法。我们已经实现了一个有效的对映特异性合成(−)-cuspareine从已知的关键起始材料。用于单个转化的反应简单且产量高,并且该策略可能很容易扩展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
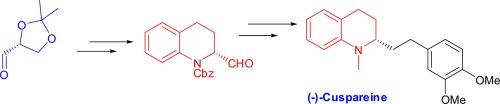
A convergent approach towards the synthesis of the 2-alkyl-substituted tetrahydroquinoline alkaloid (−)-cuspareine
A convergent approach towards the synthesis of the 2-alkyl-substituted tetrahydroquinoline alkaloid (−)-cuspareine via enantiospecific construction of the (R)-benzyl 2-formyl-3,4-dihydroquinoline-1(2H)-carboxylate. We have achieved an efficient enantiospecific synthesis of (−)-cuspareine starting from known key starting materials. The reactions employed for individual transformations are simple and high yielding, and the strategy could potentially be easily extended.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


