手性氮啶醇促进锌离子的高度对映选择性不对称反应
Q2 Chemistry
引用次数: 10
摘要
对映体纯度高的手性仲、叔氮吡啶醇(包括邻酚-偶氮酚的氮吡啶类似物)已被证明是锌离子存在下的对映选择性不对称反应的高效催化剂,包括芳香醛的芳基化、查尔酮的环氧化和二乙基锌对醛的加成,从而产生高化学收率(高达90%)和ee高达90%的理想手性产物。在上述不对称转化中,已证明了丙酚型双(氮吡啶醇)具有较高的催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
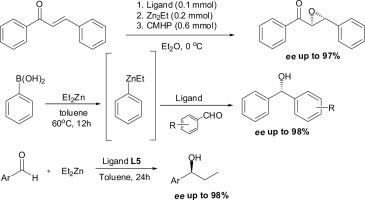
Highly enantioselective asymmetric reactions involving zinc ions promoted by chiral aziridine alcohols
Enantiomerically pure, chiral secondary and tertiary aziridine alcohols (including the aziridine analogue of ProPhenol—AziPhenol) have proven to be highly effective catalysts for enantioselective asymmetric reactions in the presence of zinc ions, including arylation of aromatic aldehydes, epoxidation of chalcone and addition of diethylzinc to aldehydes, leading to the desired chiral products in high chemical yields (up to 90%) and with ee’s up to 90%. A higher catalytic activity of Prophenol-type bis(aziridine alcohol) in the aforementioned asymmetric transformations has been demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


