致癌物质 HBXIP 诱导的 PD-L1 对乳腺癌生长的调节作用
IF 6.9
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 4
摘要
程序性死亡配体-1(PD-L1)/PD-1 检查点广泛充当免疫抑制的核心介质。研究发现,在多种癌症中,丰富的 PD-L1 具有促进肿瘤生长的作用。然而,PD-L1在乳腺癌中的重要性以及如何控制PD-L1的表达仍不清楚。本文介绍了在转录和蛋白质乙酰化水平上控制 PD-L1 促进乳腺癌生长的机制。过表达的 PD-L1 会加速乳腺癌在体外和体内的生长。RNA-seq发现PD-L1能诱导一些靶基因,影响许多细胞过程,尤其是癌症的发生发展。在临床乳腺癌组织和细胞中,PD-L1 和 HBXIP 都会增高,并且它们的表达呈正相关。机制研究发现,HBXIP 通过共同激活 ETS2 来刺激 PD-L1 的转录。具体来说,HBXIP 通过与乙酰转移酶 p300 相互作用,诱导 PD-L1 在 K270 位点乙酰化,从而导致 PD-L1 蛋白的稳定。从功能上讲,消耗 HBXIP 可减轻 PD-L1 加速的乳腺肿瘤生长。阿司匹林通过靶向 PD-L1 和 HBXIP 来缓解乳腺癌。总之,这些研究结果为了解肿瘤 PD-L1 的控制机制提供了新的思路,并拓宽了 PD-L1 作为乳腺癌治疗靶点的用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
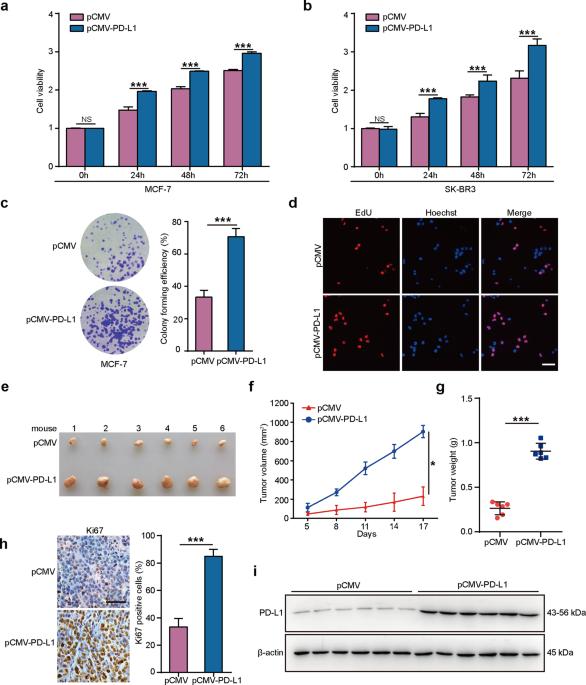
The modulation of PD-L1 induced by the oncogenic HBXIP for breast cancer growth
Programmed death ligand-1 (PD-L1)/PD-1 checkpoint extensively serves as a central mediator of immunosuppression. A tumor-promoting role for abundant PD-L1 in several cancers is revealed. However, the importance of PD-L1 and how the PD-L1 expression is controlled in breast cancer remains obscure. Here, the mechanisms of controlling PD-L1 at the transcription and protein acetylation levels in promoting breast cancer growth are presented. Overexpressed PD-L1 accelerates breast cancer growth in vitro and in vivo. RNA-seq uncovers that PD-L1 can induce some target genes affecting many cellular processes, especially cancer development. In clinical breast cancer tissues and cells, PD-L1 and HBXIP are both increased, and their expressions are positively correlated. Mechanistic exploration identifies that HBXIP stimulates the transcription of PD-L1 through co-activating ETS2. Specifically, HBXIP induces PD-L1 acetylation at K270 site through interacting with acetyltransferase p300, leading to the stability of PD-L1 protein. Functionally, depletion of HBXIP attenuates PD-L1-accelerated breast tumor growth. Aspirin alleviates breast cancer via targeting PD-L1 and HBXIP. Collectively, the findings display new light into the mechanisms of controlling tumor PD-L1 and broaden the utility for PD-L1 as a target in breast cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmacologica Sinica
医学-化学综合
CiteScore
15.10
自引率
2.40%
发文量
4365
审稿时长
2 months
期刊介绍:
APS (Acta Pharmacologica Sinica) welcomes submissions from diverse areas of pharmacology and the life sciences. While we encourage contributions across a broad spectrum, topics of particular interest include, but are not limited to: anticancer pharmacology, cardiovascular and pulmonary pharmacology, clinical pharmacology, drug discovery, gastrointestinal and hepatic pharmacology, genitourinary, renal, and endocrine pharmacology, immunopharmacology and inflammation, molecular and cellular pharmacology, neuropharmacology, pharmaceutics, and pharmacokinetics. Join us in sharing your research and insights in pharmacology and the life sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


