一种GLP-1类似物可降低ER应激并促进蛋白质折叠,从而改善同型半胱氨酸诱导的内皮功能障碍
IF 6.9
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 16
摘要
高同型半胱氨酸血症(HHcy)是心血管疾病的独立风险因素,会增加 2 型糖尿病患者的死亡率。HHcy 会诱导内质网(ER)应激和氧化应激,从而损害内皮功能。胰高血糖素样肽1(GLP-1)类似物exendin-4可减轻内皮ER应激,但血管保护机制的具体机制仍不明确。本研究探讨了 exendin-4 对 HHcy 诱导的内皮功能障碍的有益影响。Exendin-4 可逆转同型半胱氨酸诱导的 C57BL/6 小鼠主动脉体外内皮依赖性松弛功能损伤。皮下注射外显素-4四周后,从饮食诱导的HHcy小鼠体内分离出的主动脉和肠系膜动脉中受损的内皮功能均得到恢复。Exendin-4 可降低体内外小鼠主动脉中超氧化物阴离子的积累。Exendin-4 降低了人脐静脉内皮细胞(HUVECs)中ER应激标记物(如ATF4、剪接的XBP1和磷酸化的eIF2α)的表达,而这种变化在同时使用化合物C(CC)(AMPK抑制剂)处理后被逆转。Exendin-4 在 HUVECs 和动脉中诱导 AMPK 和内皮一氧化氮合酶的磷酸化。Exendin-4增加了内质网氧化还原酶(ERO1α)的表达,ER是内皮细胞中一种重要的ER伴侣蛋白,这种效应是由AMPK激活介导的。使用 siRNA 介导的基因敲除或腺病毒过表达的实验表明,ERO1α 介导了 exendin-4 对 ER 应激和超氧阴离子产生的抑制作用,从而改善了 HHcy 诱导的内皮功能障碍。本研究结果表明,依托于 AMPK 的ERO1α在内皮细胞和动脉中上调,外显素-4 可降低 HHcy 诱导的 ER 应激,改善内皮功能。AMPK 激活可促进内皮细胞中的蛋白质折叠机制,从而抑制 ER 应激。本文章由计算机程序翻译,如有差异,请以英文原文为准。
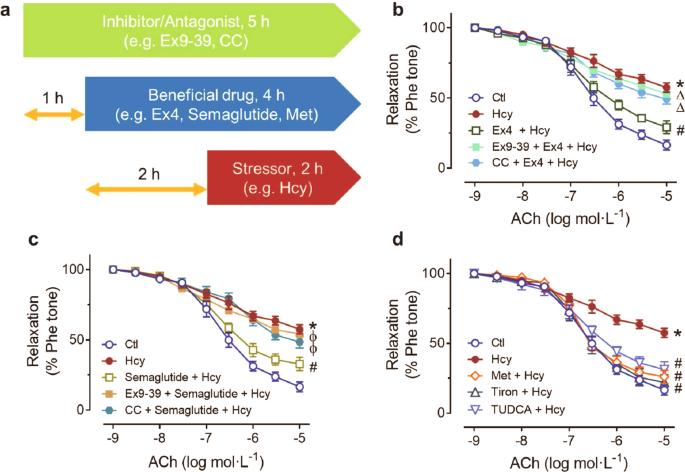
A GLP-1 analog lowers ER stress and enhances protein folding to ameliorate homocysteine-induced endothelial dysfunction
Hyperhomocysteinemia (HHcy) is an independent risk factor for cardiovascular diseases and increases mortality in type 2 diabetic patients. HHcy induces endoplasmic reticulum (ER) stress and oxidative stress to impair endothelial function. The glucagon-like peptide 1 (GLP-1) analog exendin-4 attenuates endothelial ER stress, but the detailed vasoprotective mechanism remains elusive. The present study investigated the beneficial effects of exendin-4 against HHcy-induced endothelial dysfunction. Exendin-4 pretreatment reversed homocysteine-induced impairment of endothelium-dependent relaxations in C57BL/6 mouse aortae ex vivo. Four weeks subcutaneous injection of exendin-4 restored the impaired endothelial function in both aortae and mesenteric arteries isolated from mice with diet-induced HHcy. Exendin-4 treatment lowered superoxide anion accumulation in the mouse aortae both ex vivo and in vivo. Exendin-4 decreased the expression of ER stress markers (e.g., ATF4, spliced XBP1, and phosphorylated eIF2α) in human umbilical vein endothelial cells (HUVECs), and this change was reversed by cotreatment with compound C (CC) (AMPK inhibitor). Exendin-4 induced phosphorylation of AMPK and endothelial nitric oxide synthase in HUVECs and arteries. Exendin-4 increased the expression of endoplasmic reticulum oxidoreductase (ERO1α), an important ER chaperone in endothelial cells, and this effect was mediated by AMPK activation. Experiments using siRNA-mediated knockdown or adenoviral overexpression revealed that ERO1α mediated the inhibitory effects of exendin-4 on ER stress and superoxide anion production, thus ameliorating HHcy-induced endothelial dysfunction. The present results demonstrate that exendin-4 reduces HHcy-induced ER stress and improves endothelial function through AMPK-dependent ERO1α upregulation in endothelial cells and arteries. AMPK activation promotes the protein folding machinery in endothelial cells to suppress ER stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmacologica Sinica
医学-化学综合
CiteScore
15.10
自引率
2.40%
发文量
4365
审稿时长
2 months
期刊介绍:
APS (Acta Pharmacologica Sinica) welcomes submissions from diverse areas of pharmacology and the life sciences. While we encourage contributions across a broad spectrum, topics of particular interest include, but are not limited to: anticancer pharmacology, cardiovascular and pulmonary pharmacology, clinical pharmacology, drug discovery, gastrointestinal and hepatic pharmacology, genitourinary, renal, and endocrine pharmacology, immunopharmacology and inflammation, molecular and cellular pharmacology, neuropharmacology, pharmaceutics, and pharmacokinetics. Join us in sharing your research and insights in pharmacology and the life sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


