喹啉-4(1 H)- 1首次立体选择性合成二乙基顺式和反式(4-羟基-1,2,3,4-四氢喹啉-2-基)膦酸酯和苯基膦酸酯
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种立体选择性合成新型二乙基顺式和反式(4-羟基-1,2,3,4-四氢喹啉-2-基)膦酸盐,以及乙基顺式和反式(4-羟基-1,2,3,4-四氢喹啉-2-基)苯基膦酸盐的实用方法。该方法的主要特点是用亚磷酸二乙酯或苯基膦酸乙酯进行区域选择性的1,4-磷酰化,得到N-Cbz喹啉-4(1H)- 1,然后进行高非对映选择性还原,得到顺式立体异构体作为有利产物,通过Mitsunobu反应转化为反式立体异构体。在氢解作用下,N-Cbz键断裂,得到目标杂环α-氨基膦酸盐和α-氨基膦酸盐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
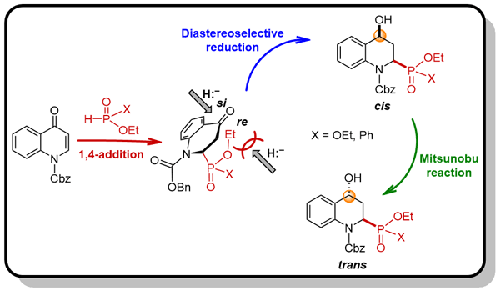
First Stereoselective Synthesis of Diethyl cis - and trans -(4-Hydroxy-1,2,3,4-tetrahydroquinolin-2-yl)phosphonates and Ethyl Phenylphosphinates from Quinolin-4(1 H )-one
We report here a practical method for the first stereoselective synthesis of novel diethyl cis- and trans-(4-hydroxy-1,2,3,4-tetrahydro-quinolin-2-yl)phosphonates, as well as the ethyl cis- and trans-(4-hydroxy-1,2,3,4-tetrahydroquinolin-2-yl)phenylphosphinates. The main feature of this method involves the regioselective 1,4-phosponylation to N-Cbz quinolin-4(1H)-one using diethyl phosphite or ethyl phenylphosphinate followed by the high diastereoselective reduction, to give the cis stereoisomers as favored products, which through the Mitsunobu reaction were converted into trans stereoisomers. Cleavage of N-Cbz bond under hydrogenolysis, gave the target heterocyclic α-aminophosphonates and α-aminophosphinates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


