Hederacoside C 通过调节 S100A9/MAPK 和中性粒细胞招募失活,恢复受损的肠道屏障,从而改善结肠炎
IF 6.9
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 2
摘要
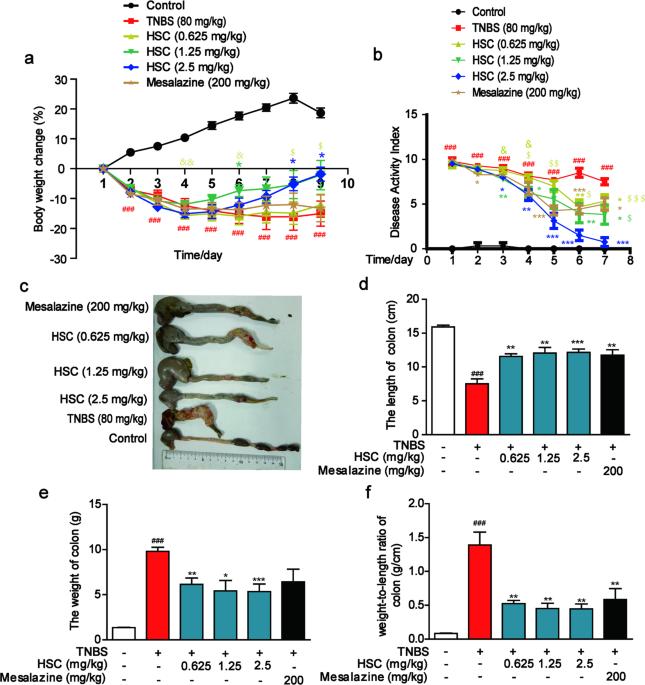
Hederacoside C(HSC)作为一种新型炎症调节剂备受关注,但其抗炎机理仍然难以捉摸。在本研究中,我们研究了 HSC 如何在体内和体外减轻肠道炎症。造血干细胞注射通过抑制促炎细胞因子的产生和结肠上皮细胞的凋亡,明显缓解了 TNBS 引起的结肠炎,并部分恢复了结肠上皮细胞的增殖。注射造血干细胞的治疗效果与口服美沙拉嗪(200 mg-kg-1-d-1,i.g.)相当。在 LPS 刺激的人肠道上皮 Caco-2 细胞中,HSC(0.1、1、10 μM)的预处理能显著抑制 MAPK/NF-κB 及其下游信号通路的激活。用 HSC 预处理可防止 LPS 诱导的 TLR4 二聚化和 MyD88 体外招募。定量蛋白质组分析表明,注射 HSC 能调节结肠样本中的 18 种蛋白质,主要集中在中性粒细胞脱颗粒中。其中,参与中性粒细胞脱颗粒的S100A9是下调最明显的蛋白之一。HSC抑制了结肠中S100A9及其下游基因(包括TLR4、MAPK和NF-κB轴)的表达。在Caco-2细胞中,重组S100A9蛋白激活了MAPK/NF-κB信号通路并诱发炎症,而使用HSC预处理后,炎症有所缓解。值得注意的是,造血干细胞在体外和体内都能减少中性粒细胞的募集和脱颗粒以及 S100A9 的释放。此外,造血干细胞还能促进紧密连接蛋白的表达,并通过抑制 S100A9 修复上皮屏障。我们的研究结果验证了造血干细胞可通过抑制S100A9/MAPK和中性粒细胞招募失活来修复受损的肠屏障,从而改善结肠炎,这表明造血干细胞是一种治疗结肠炎的有前途的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hederacoside C ameliorates colitis via restoring impaired intestinal barrier through moderating S100A9/MAPK and neutrophil recruitment inactivation
Hederacoside C (HSC) has attracted much attention as a novel modulator of inflammation, but its anti-inflammatory mechanism remains elusive. In the present study, we investigated how HSC attenuated intestinal inflammation in vivo and in vitro. HSC injection significantly alleviated TNBS-induced colitis by inhibiting pro-inflammatory cytokine production and colonic epithelial cell apoptosis, and partially restored colonic epithelial cell proliferation. The therapeutic effect of HSC injection was comparable to that of oral administration of mesalazine (200 mg·kg−1·d−1, i.g.). In LPS-stimulated human intestinal epithelial Caco-2 cells, pretreatment with HSC (0.1, 1, 10 μM) significantly inhibited activation of MAPK/NF-κB and its downstream signaling pathways. Pretreatment with HSC prevented LPS-induced TLR4 dimerization and MyD88 recruitment in vitro. Quantitative proteomic analysis revealed that HSC injection regulated 18 proteins in the colon samples, mainly clustered in neutrophil degranulation. Among them, S100A9 involved in the degranulation of neutrophils was one of the most significantly down-regulated proteins. HSC suppressed the expression of S100A9 and its downstream genes including TLR4, MAPK, and NF-κB axes in colon. In Caco-2 cells, recombinant S100A9 protein activated the MAPK/NF-κB signaling pathway and induced inflammation, which were ameliorated by pretreatment with HSC. Notably, HSC attenuated neutrophil recruitment and degranulation as well as S100A9 release in vitro and in vivo. In addition, HSC promoted the expression of tight junction proteins and repaired the epithelial barrier via inhibiting S100A9. Our results verify that HSC ameliorates colitis via restoring impaired intestinal barrier through moderating S100A9/MAPK and neutrophil recruitment inactivation, suggesting that HSC is a promising therapeutic candidate for colitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmacologica Sinica
医学-化学综合
CiteScore
15.10
自引率
2.40%
发文量
4365
审稿时长
2 months
期刊介绍:
APS (Acta Pharmacologica Sinica) welcomes submissions from diverse areas of pharmacology and the life sciences. While we encourage contributions across a broad spectrum, topics of particular interest include, but are not limited to: anticancer pharmacology, cardiovascular and pulmonary pharmacology, clinical pharmacology, drug discovery, gastrointestinal and hepatic pharmacology, genitourinary, renal, and endocrine pharmacology, immunopharmacology and inflammation, molecular and cellular pharmacology, neuropharmacology, pharmaceutics, and pharmacokinetics. Join us in sharing your research and insights in pharmacology and the life sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


