拟南芥synaptotagmin 1以脂质成分依赖的方式介导脂质转运
IF 2.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 6
摘要
真核生物的内质网(ER) -质膜(PM)接触位点(EPCSs)在结构上是保守的。拟南芥ER锚定突触蛋白1 (SYT1)在epcs中富集,在植物非生物胁迫耐受中起关键作用。现在已经很清楚,SYT1与PM相互作用介导ER - PM连接。然而,SYT1是否在epcs中执行其他功能仍然未知。在这里,我们报道SYT1有效地在膜之间转移磷脂。SYT1的脂质转移活性高度依赖于磷脂酰肌醇4,5 -二磷酸[PI(4,5)P2],这是一种在非生物胁迫下在PM积累的信号脂质。从机械上讲,SYT1通过synaptotagmin - like线粒体脂质结合蛋白(SMP)结构域转移脂质,而有效的脂质运输需要C2A结构域介导的膜系固。有趣的是,我们观察到Ca2+可以刺激SYT1介导的脂质转运。除了PI(4,5)P2外,Ca2+的激活还需要磷脂酰丝氨酸,这是另一种在对立膜上带负电荷的脂质。总之,我们的研究确定拟南芥SYT1是EPCSs中的脂质转移蛋白,并证明它与其他延伸突触tagmins具有保守和不同的机制。脂质组成和Ca2+的关键作用表明,SYT1介导的脂质转运在响应非生物胁迫的信号中受到高度调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
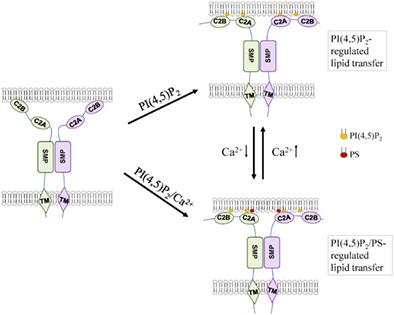
Arabidopsis synaptotagmin 1 mediates lipid transport in a lipid composition‐dependent manner
The endoplasmic reticulum (ER)‐plasma membrane (PM) contact sites (EPCSs) are structurally conserved in eukaryotes. The Arabidopsis ER‐anchored synaptotagmin 1 (SYT1), enriched in EPCSs, plays a critical role in plant abiotic stress tolerance. It has become clear that SYT1 interacts with PM to mediate ER‐PM connectivity. However, whether SYT1 performs additional functions at EPCSs remains unknown. Here, we report that SYT1 efficiently transfers phospholipids between membranes. The lipid transfer activity of SYT1 is highly dependent on phosphatidylinositol 4,5‐bisphosphate [PI(4,5)P2], a signal lipid accumulated at the PM under abiotic stress. Mechanically, while SYT1 transfers lipids fundamentally through the synaptotagmin‐like mitochondrial‐lipid‐binding protein (SMP) domain, the efficient lipid transport requires the C2A domain‐mediated membrane tethering. Interestingly, we observed that Ca2+ could stimulate SYT1‐mediated lipid transport. In addition to PI(4,5)P2, the Ca2+ activation requires the phosphatidylserine, another negatively charged lipid on the opposed membrane. Together, our studies identified Arabidopsis SYT1 as a lipid transfer protein at EPCSs and demonstrated that it takes conserved as well as divergent mechanisms with other extend‐synaptotagmins. The critical role of lipid composition and Ca2+ reveals that SYT1‐mediated lipid transport is highly regulated by signals in response to abiotic stresses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Traffic
生物-细胞生物学
CiteScore
8.10
自引率
2.20%
发文量
50
审稿时长
2 months
期刊介绍:
Traffic encourages and facilitates the publication of papers in any field relating to intracellular transport in health and disease. Traffic papers span disciplines such as developmental biology, neuroscience, innate and adaptive immunity, epithelial cell biology, intracellular pathogens and host-pathogen interactions, among others using any eukaryotic model system. Areas of particular interest include protein, nucleic acid and lipid traffic, molecular motors, intracellular pathogens, intracellular proteolysis, nuclear import and export, cytokinesis and the cell cycle, the interface between signaling and trafficking or localization, protein translocation, the cell biology of adaptive an innate immunity, organelle biogenesis, metabolism, cell polarity and organization, and organelle movement.
All aspects of the structural, molecular biology, biochemistry, genetics, morphology, intracellular signaling and relationship to hereditary or infectious diseases will be covered. Manuscripts must provide a clear conceptual or mechanistic advance. The editors will reject papers that require major changes, including addition of significant experimental data or other significant revision.
Traffic will consider manuscripts of any length, but encourages authors to limit their papers to 16 typeset pages or less.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


