混合碳羧酸酰胺化羧酸及其在抗抑郁药(1S,2R)-丙氨酰环丙氨酸合成中的应用
Q2 Chemistry
引用次数: 9
摘要
在ClCO2Et和Et3N存在下,用NH4Cl制备羧酸1或羧酸3的伯胺,得到相应的伯胺,产率为22%。此外,我们将酰胺化应用于制备各种含羟肟酸的酰胺,并通过Lossen重排合成了(1S,2R)-丙氨酰环丙胺作为抗抑郁药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
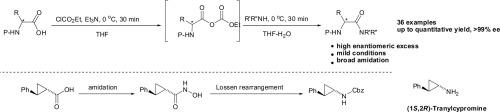
Amidation of carboxylic acids via the mixed carbonic carboxylic anhydrides and its application to synthesis of antidepressant (1S,2R)-tranylcypromine
Primary amidations of carboxylic acids 1 or 3 with NH4Cl in the presence of ClCO2Et and Et3N were developed to afford the corresponding primary amides in 22% to quantitative yields. Additionally, we have applied the amidation to the preparation of various amides containing hydroxamic acids and achieved the synthesis of (1S,2R)-tranylcypromine as an antidepressant medicine via Lossen rearrangement.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


