一种基于手性双环骨架的对映纯二硒化物-合成和构型分配
Q2 Chemistry
引用次数: 1
摘要
从各自的卤化物制备了对映体纯的含两个手性双环亚基的二硒化物。通过核磁共振波谱和x射线衍射测量确定了反应的立体化学结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
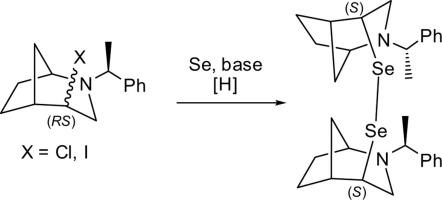
An enantiopure diselenide based on a chiral bicyclic backbone—synthesis and configuration assignment
An enantiomerically pure diselenide containing two chiral bicyclic subunits was prepared from the respective halides. The stereochemical outcome of the reaction was established via NMR spectroscopy and X-ray diffraction measurements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


