脂肪酶介导的顺式和反式芳樟醇氧化物(pyranoid)的分解
Q2 Chemical Engineering
引用次数: 8
摘要
以廉价的单萜萜烯为原料,合成了四种异构体形式的芳樟醇氧化物(类吡喃酮)。该工艺的关键步骤包括起始二烯的mCPBA环氧化,得到的二氧化物的铝催化重排和酮2,2,6-三甲基-6-乙烯基二氢- 2h -吡喃-3(4H)- 1的非对映选择性还原。所得到的外消旋顺式和反式芳樟醇氧化物通过酶介导的乙酰化过程被分解。更具体地说,我们发现念珠菌脂肪酶和脂肪酶PS分别是分解顺式和反式芳樟醇氧化物的催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
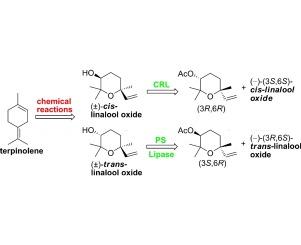
Lipase mediated resolution of cis- and trans-linalool oxide (pyranoid)
The four isomeric forms of the flavour linalool oxide (pyranoid) were synthesized starting from the inexpensive monoterpene terpinolene. The key steps of the process include mCPBA epoxidation of the starting diene, alumina-catalysed rearrangement of the obtained diepoxide and the diastereoselective reduction of the ketone 2,2,6-trimethyl-6-vinyldihydro-2H-pyran-3(4H)-one. The resulting racemic cis- and trans-linalool oxides were resolved through an enzyme-mediated acetylation procedure. More specifically, we found that Candida rugosa lipase and lipase PS are the catalysts of choice for the resolution of cis- and trans-linalool oxide, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Catalysis B-enzymatic
生物-生化与分子生物学
CiteScore
2.58
自引率
0.00%
发文量
0
审稿时长
3.4 months
期刊介绍:
Journal of Molecular Catalysis B: Enzymatic is an international forum for researchers and product developers in the applications of whole-cell and cell-free enzymes as catalysts in organic synthesis. Emphasis is on mechanistic and synthetic aspects of the biocatalytic transformation.
Papers should report novel and significant advances in one or more of the following topics;
Applied and fundamental studies of enzymes used for biocatalysis;
Industrial applications of enzymatic processes, e.g. in fine chemical synthesis;
Chemo-, regio- and enantioselective transformations;
Screening for biocatalysts;
Integration of biocatalytic and chemical steps in organic syntheses;
Novel biocatalysts, e.g. enzymes from extremophiles and catalytic antibodies;
Enzyme immobilization and stabilization, particularly in non-conventional media;
Bioprocess engineering aspects, e.g. membrane bioreactors;
Improvement of catalytic performance of enzymes, e.g. by protein engineering or chemical modification;
Structural studies, including computer simulation, relating to substrate specificity and reaction selectivity;
Biomimetic studies related to enzymatic transformations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


