TCFH-NMI:直接获得n -酰基咪唑以挑战酰胺键形成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 54
摘要
利用N,N,N ',N ' -四甲基氯甲脒六氟磷酸(TCFH)和N-甲基咪唑(NMI)的组合,阻碍羧酸与非亲核胺形成酰胺键的具有挑战性的偶联可以在高收率下完成,并且在许多情况下,可以完全保留相邻的立体中心。这种方法允许在原位生成高活性的酰基咪唑离子,这已被证明是反应的中间体。该试剂提供高反应性类似于酸性氯化物与易于使用的现代铀试剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
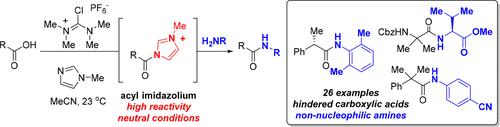
TCFH–NMI: Direct Access to N-Acyl Imidazoliums for Challenging Amide Bond Formations
Challenging couplings of hindered carboxylic acids with non-nucleophilic amines to form amide bonds can be accomplished in high yields, and in many cases, with complete retention of the adjacent stereogenic centers using the combination of N,N,N′,N′-tetramethylchloroformamidinium hexafluorophosphate (TCFH) and N-methylimidazole (NMI). This method allows for in situ generation of highly reactive acyl imidazolium ions, which have been demonstrated to be intermediates in the reaction. The reagent delivers high reactivity similar to acid chlorides with the ease of use of modern uronium reagents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


