β-酮酸脱羧缩合合成γ-吡咯酮。
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
摘要:本文报道了以β-酮酸为原料会聚合成芳基和烷基二取代γ-吡咯酮。在三氟甲烷磺酸酐的存在下,反应通过前所未有的起始物质的脱羧自缩合进行。在此,报告了这种转换的范围和局限性。图形化的简介:本文章由计算机程序翻译,如有差异,请以英文原文为准。
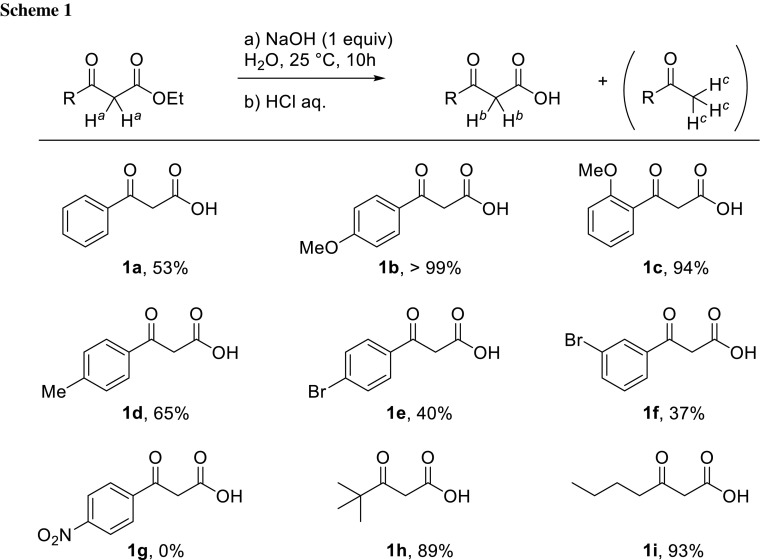
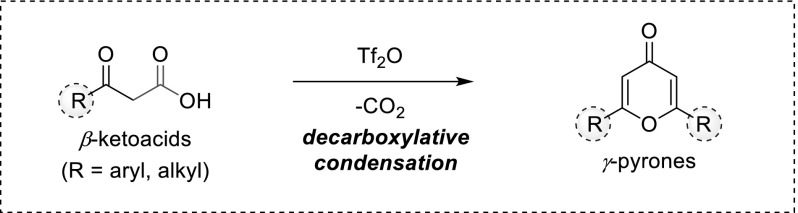
Synthesis of γ-pyrones via decarboxylative condensation of β-ketoacids.
Abstract: This manuscript describes the convergent synthesis of aryl- and alkyl-disubstituted γ-pyrones from β-ketoacids. The reaction proceeds in the presence of trifluoromethanesulfonic anhydride via an unprecedented decarboxylative auto-condensation of the starting material. Herein, the scope and limitations of this transformation are reported.
Graphical abstract:
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Monatshefte Fur Chemie
化学-化学综合
CiteScore
3.70
自引率
5.60%
发文量
116
审稿时长
3.3 months
期刊介绍:
"Monatshefte für Chemie/Chemical Monthly" was originally conceived as an Austrian journal of chemistry. It has evolved into an international journal covering all branches of chemistry. Featuring the most recent advances in research in analytical chemistry, biochemistry, inorganic, medicinal, organic, physical, structural, and theoretical chemistry, Chemical Monthly publishes refereed original papers and a section entitled "Short Communications". Reviews, symposia in print, and issues devoted to special fields will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


