糖基三唑吡啶酰胺/ cu催化2-卤代苯酰胺与活性亚甲基化合物的偶联
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种在高效的糖基1,2,3-三唑吡啶酰胺配体辅助下,Cu(I)催化串联合成二氢苯基二酮和取代异喹啉酮的简便方法。该催化体系可有效地将n -取代2-卤代苯酰胺与多种活性亚甲基化合物偶联,从而获得高至优异的生物相关杂环支架收率。反应的连续路径包括分子间的C-C交叉偶联,然后是分子内的环化,在低催化负荷下有效地发生。通过CuI/DIPEA介导的区域选择性CuAAC点击工具,以较好的收率合成了这些糖基三唑附加吡啶酰胺。该方法有一些显著的特点,包括低催化负荷,配体的成本效益和生物相容性,高反应产率,以及易于获取的起始材料,使该方案更加通用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
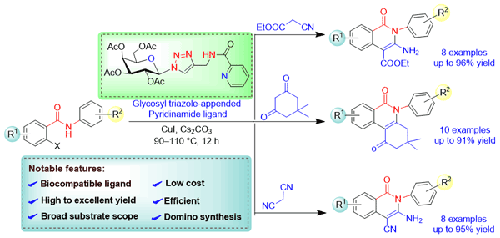
Glycosyl Triazole Based Pyridinamide/CuI-Catalyzed Coupling of 2-Halobenzamides with Active Methylene Compounds
The report describes a convenient method for the Cu(I)-catalyzed tandem synthesis of dihydrophenanthridine-diones and substituted isoquinolinones with the assistance of efficient glycosyl 1,2,3-triazole-based pyridinamide ligands. The catalytic system effectively works for the coupling of N-substituted 2-halobenzamides with various active methylene compounds to achieve a high-to-excellent yield of biologically relevant heterocyclic scaffolds. The consecutive path of the reaction including intermolecular C-C cross-coupling followed by intramolecular cyclization efficiently takes place at low catalytic loading. These glycosyl triazole-appended pyridinamides were synthesized in good yields via CuI/DIPEA mediated regioselective CuAAC click tool. There are some notable features of the method, including low catalytic loading, the cost-effective and biocompatible nature of ligands, high reaction yield, and easily accessible starting materials that make the protocol more versatile.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


