黑坎宁哈默菌生物转化雄激素药物美睾酮的三种新类似物
Q2 Chemical Engineering
引用次数: 4
摘要
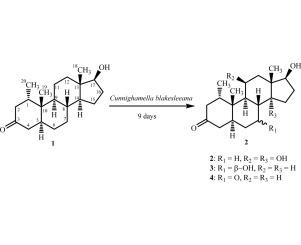
雄激素甾体酮(1)与黑坎宁哈默氏菌孵育得到3个新的代谢物。这些代谢产物鉴定为1α-甲基-11β,14α,17β-三羟基-5α-雄酮-3-酮(2),1α-甲基-7β,17β-二羟基-5α-雄酮-3-酮(3)和1α-甲基,17β-羟基-5α-雄酮-3,7-二酮(4)。在本研究中,观察到底物1在C-11, C-14和C-15位点的羟基化和C-7位点的氧化。C-11位点的β-羟基化是C. blakesleeana的一个相当独特的转化,因为α-羟基化据报道可以被大多数其他微生物催化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Three new analogues of androgenic drug mesterolone through biotransformation with Cunninghamella blakseleeana
Three new metabolites were obtained on incubation of androgenic steroid mesterolone (1) with Cunninghamella blakesleeana. These metabolites were identified as 1α-methyl-11β,14α,17β-trihydroxy-5α-androstan-3-one (2), 1α-methyl-7β,17β-dihydroxy-5α-androstan-3-one (3), and 1α-methyl,17β-hydroxy-5α-androstan-3,7-dione (4). During this study, hydroxylation at C-11, C-14, and C-15, and oxidation at C-7 of substrate 1 were observed. β-Hydroxylation at C-11 is a rather unique transformation by C. blakesleeana, as α-hydroxylation is reported to be catalyzed by most of the other microorganisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Catalysis B-enzymatic
生物-生化与分子生物学
CiteScore
2.58
自引率
0.00%
发文量
0
审稿时长
3.4 months
期刊介绍:
Journal of Molecular Catalysis B: Enzymatic is an international forum for researchers and product developers in the applications of whole-cell and cell-free enzymes as catalysts in organic synthesis. Emphasis is on mechanistic and synthetic aspects of the biocatalytic transformation.
Papers should report novel and significant advances in one or more of the following topics;
Applied and fundamental studies of enzymes used for biocatalysis;
Industrial applications of enzymatic processes, e.g. in fine chemical synthesis;
Chemo-, regio- and enantioselective transformations;
Screening for biocatalysts;
Integration of biocatalytic and chemical steps in organic syntheses;
Novel biocatalysts, e.g. enzymes from extremophiles and catalytic antibodies;
Enzyme immobilization and stabilization, particularly in non-conventional media;
Bioprocess engineering aspects, e.g. membrane bioreactors;
Improvement of catalytic performance of enzymes, e.g. by protein engineering or chemical modification;
Structural studies, including computer simulation, relating to substrate specificity and reaction selectivity;
Biomimetic studies related to enzymatic transformations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


