香叶醇和橙醇基香叶醇对映体的合成及立体化学配位
Q2 Chemistry
引用次数: 1
摘要
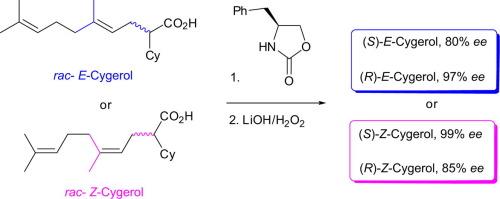
通过低温烷基化、碱性水解、(S)-4-苯氧苄唑烷酮-2- 1衍生化和色谱分离等步骤,制备了香叶醇和橙醇衍生的2-环己基-5,9-二甲基十二-4,8-二烯酸对映体(ee达99%)。根据相应的(S)-4-苯并氯唑烷-2- 1衍生亚胺的特征1H NMR信号,以及已知的(S)-2-环己基琥珀酸二乙基和(S)-2-环己基丁烷-1,4-二醇的转化,确定了具有(E)-或(Z)-几何结构的内双键对映体中立体中心的绝对构型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and stereochemical assignment of geraniol- and nerol-derived Cygerol enantiomers
The enantiomers (up to 99% ee) of both geraniol- and nerol-derived 2-cyclohexyl-5,9-dimethyldeca-4,8-dienoic acid, the active ingredient of the wound healing medication Cygerol, were prepared via a low-temperature alkylation, basic hydrolysis, derivatization with (S)-4-benzyloxazolidin-2-one and chromatographic separation steps. The absolute configuration of stereocenters in the antipodes having an (E)- or (Z)-geometry of the internal double bond was determined based on characteristic 1H NMR signals of the corresponding (S)-4-benzyloxazolidin-2-one-derived imides and on conversion to the known diethyl (S)-2-cyclohexylsuccinate and (S)-2-cyclohexylbutane-1,4-diol with reported specific rotations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


