n -叔丁烷磺酸基-α-酮醛胺的立体选择性烯丙基化和还原
Q2 Chemistry
引用次数: 8
摘要
本文提出了一种由α-酮醛和羧酸酯合成n -叔丁烷磺酸基α-酮醛胺的简单方法。还研究了在这些手性亚胺中加入原位形成的烯丙基铟试剂。加成是依次发生的,首先是对非对映选择性好的亚胺基团,然后是对非对映选择性较低的羰基。钌催化的5-氨基乙酸-1,7-二烯-4-醇衍生物的合环复合反应可获得6-氨基环己烯醇。α-酮醛胺的还原得到n -叔丁烷磺酸基-1,2-氨基醇为1:1的非对映体混合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
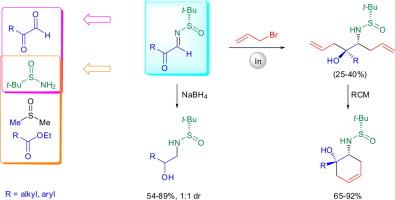
Stereoselective allylation and reduction of N-tert-butanesulfinyl-α-keto aldimines
A simple methodology for the synthesis of N-tert-butanesulfinyl-α-keto aldimines from both α-keto aldehydes and carboxylic esters has been developed. The addition of an in situ formed allyl indium reagent to these chiral imines was also studied. The addition took place in a sequential manner, first to the imine group with excellent diastereoselectivity and then to the carbonyl group with lower diastereoselectivity. Ruthenium-catalyzed ring closing metathesis of the resulting 5-aminoocta-1,7-dien-4-ol derivatives provided access to 6-aminocyclohex-3-enols. Reduction of the α-keto aldimines led to N-tert-butanesulfinyl-1,2-aminoalcohols as a 1:1 diastereomeric mixture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


