镍催化环丙胺和其他张力环NHP酯与(杂)芳基卤化物的还原交偶联
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 5
摘要
报道了镍催化环丙胺NHP酯与(杂)芳基卤化物的还原交偶联反应。这种高效的方案提供了1-芳基环丙胺的直接访问,这是一种生物等构基序,通常用于小分子药物的发现。反应进行迅速(2小时),具有良好的官能团耐受性,不需要热敏或气敏试剂。该方法也可推广到四元应变环的芳基化。从相应的市售羧酸中一步得到NHP酯,收率高,无需柱层析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
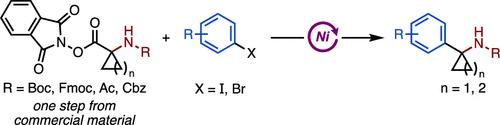
Ni-Catalyzed Reductive Cross-Coupling of Cyclopropylamines and Other Strained Ring NHP Esters with (Hetero)Aryl Halides
A nickel-catalyzed reductive cross-coupling of cyclopropylamine NHP esters with (hetero)aryl halides is reported. This efficient protocol provides direct access to 1-arylcyclopropylamines, a bioisosteric motif commonly used in small molecule drug discovery. The reaction proceeds rapidly (<2 h) with excellent functional group tolerance and without requiring heat- or air-sensitive reagents. The method can also be extended to the arylation of four-membered strained rings. The NHP esters are easily obtained from the corresponding commercially available carboxylic acids in one step with high yields and no column chromatography.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


