使用可回收螯合树脂从水溶液中选择性识别和提取铀酰离子†
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
制备了一种离子交换聚合物1,该聚合物结合了针对铀酰离子设计的螯合配体,并研究了其从水溶液中去除铀的能力。螯合模块显示形成1 : 1和溶液中的铀酰离子的络合物。在铀酰提取实验中进行了1与标准亚氨基二乙酸螯合树脂Chelex 100的比较。1有效地从水溶液中提取铀酰离子,包括掺入的海水,并可完全回收至少15个提取循环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
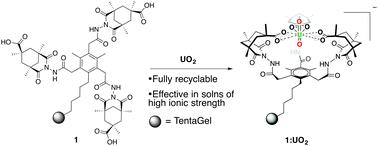
Selective recognition and extraction of the uranyl ion from aqueous solutions with a recyclable chelating resin†
An ion exchange
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


