通过使用阈值技术的在线调查对未来阿尔茨海默病治疗的偏好
IF 7.8
3区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
摘要
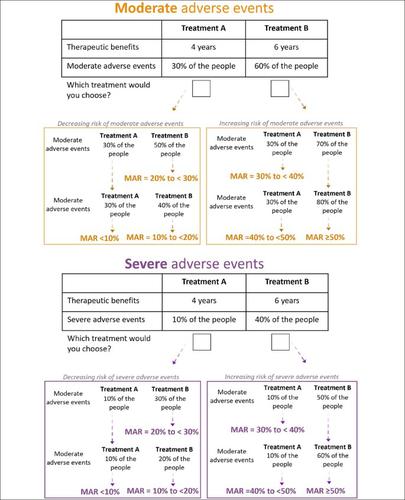
旨在减缓阿尔茨海默病(AD)进展的治疗方法可能很快就会出现。然而,人们为了延缓疾病进展而愿意接受的风险信息是有限的。确定个人愿意在AD假设治疗的益处和风险之间做出的权衡,以及这些权衡在多大程度上取决于个人的特征和对药物的信念。在线,横断面调查研究。英国的人口。在英国阿尔茨海默氏症研究和加入痴呆症研究的网站上可以找到调查的公共链接。所有自我报告≥18岁的人都有资格参加。共有4384人参与调查,3658人完成了调查。参与者发生中度和重度不良事件的最大可接受风险(MARs),以换取2年的疾病进展延迟。风险以顺序量表表示,从<10%到≥50%,高于中度不良事件存在的30%和严重不良事件存在的10%的风险。我们使用对数正态生存模型获得了人群中位MARs,并根据加速因子量化了个体特征和对药物的信念的影响。对于中度不良事件,26%的参与者的MAR≥50%,其次是25%的参与者,MAR为10至<20%,估计MAR中位数为25.4%(95%置信区间[CI] 24.5至26.3)。对于严重不良事件,43%的参与者的MAR <10%,其次是25%的参与者,MAR为10至<20%,导致估计中位MAR为12.1% (95%CI 11.6至12.5)。与个人不良事件MARs相关的因素有年龄、性别、教育水平、独居和对药物的信念。无论个人是否有记忆问题或是否有过照顾者的经历,对MARs的任何不良事件都没有影响。阿尔茨海默病治疗的益处和风险之间的权衡是异质的,并受到个体特征和对药物的信念的影响。在药品决策过程中应承认这种异质性,以满足不同亚群的需求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Preferences about Future Alzheimer’s Disease Treatments Elicited through an Online Survey Using the Threshold Technique
Treatments aiming at slowing down the progression of Alzheimer’s disease (AD) may soon become available. However, information about the risks that people are willing to accept in order to delay the progression of the disease is limited. To determine the trade-offs that individuals are willing to make between the benefits and risks of hypothetical treatments for AD, and the extent to which these trade-offs depend on individuals’ characteristics and beliefs about medicines. Online, cross-sectional survey study. Population in the UK. Public link to the survey available at the websites of Alzheimer’s Research UK and Join Dementia Research. Everyone self-reported ≥18 years old was eligible to participate. A total of 4384 people entered the survey and 3658 completed it. The maximum acceptable risks (MARs) of participants for moderate and severe adverse events in exchange for a 2-year delay in disease progression. The risks were expressed on ordinal scales, from <10% to ≥50%, above a pre-existing risk of 30% for moderate adverse events and 10% for severe adverse events. We obtained the population median MARs using log-normal survival models and quantified the effects of individuals’ characteristics and beliefs about medicines in terms of acceleration factors. For the moderate adverse events, 26% of the participants had a MAR ≥50%, followed by 25% of the participants with a MAR of 10 to <20%, giving an estimated median MAR of 25.4% (95% confidence interval [CI] 24.5 to 26.3). For the severe adverse events, 43% of the participants had a MAR <10%, followed by 25% of the participants with a MAR of 10 to <20%, resulting in an estimated median MAR of 12.1% (95%CI 11.6 to 12.5). Factors that were associated with the individuals’ MARs for one or both adverse events were age, gender, educational level, living alone, and beliefs about medicines. Whether or not individuals were living with memory problems or had experience as a caregiver had no effect on the MARs for any of the adverse events. Trade-offs between benefits and risks of AD treatments are heterogeneous and influenced by individuals’ characteristics and beliefs about medicines. This heterogeneity should be acknowledged during the medicinal product decision-making in order to fulfil the needs of the various subpopulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Jpad-Journal of Prevention of Alzheimers Disease
CLINICAL NEUROLOGY-
自引率
7.80%
发文量
85
期刊介绍:
The JPAD « Journal of Prevention of Alzheimer’Disease » will publish reviews, original research articles and short reports to improve our knowledge in the field of Alzheimer prevention including : neurosciences, biomarkers, imaging, epidemiology, public health, physical cognitive exercise, nutrition, risk and protective factors, drug development, trials design, and heath economic outcomes.
JPAD will publish also the meeting abstracts from Clinical Trial on Alzheimer Disease (CTAD) and will be distributed both in paper and online version worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


