口服多种营养素治疗干预减少衰老对认知的影响:反应试验研究设计
IF 7.8
3区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
摘要
据报道,针对阿尔茨海默病和阿尔茨海默病引起的轻度认知障碍,采用特定的多营养素干预(Souvenaid)可获得临床益处。苏维尼在与年龄相关的认知衰退中的作用尚未确定。评估使用虚拟评估来研究多种营养素对认知老化的影响的可行性。这是一项随机、双盲、安慰剂对照、平行组虚拟试点试验,在单中心进行了6个月以上。参与者被随机分配(1:1),接受特定的多营养素(Souvenaid)或等热量、相同味道的安慰剂。试用访问使用安全的在线视频通信进行虚拟。年龄在55-89岁之间、说英语或西班牙语且被认为有与年龄相关的认知能力下降的所有种族的人都符合条件。在基线和干预6个月后进行神经心理学测试。参与者每月通过电话联系,以监督安全,评估动机和促进遵守。主要结局是通过评估招募率、招募时间、依从率和保留率来确定可行性。一套全面的神经心理学测量将提供认知功能的广泛评估,包括言语记忆、处理速度、注意力和执行功能。自我报告问卷用于评估生活质量。该试点试验将提供数据,以指导未来年龄相关认知衰退研究的参与者选择和结果测量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
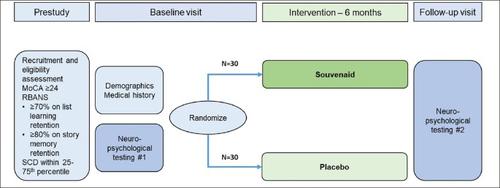
Reducing the Effects of Ageing on Cognition with Therapeutic Intervention of an Oral Multi-Nutrient: The REACTION Pilot Trial Study Design
Clinical benefits have been reported with a specific multinutrient intervention (Souvenaid) in Alzheimer’s disease and mild cognitive impairment due to Alzheimer’s disease. The effects of Souvenaid in age-related cognitive decline are not established. To assess the feasibility of using virtual assessments to study the effects of a multinutrient on cognitive ageing. This is a randomized, double-blind, placebo-controlled, parallel group virtual pilot trial performed over 6 months in a single-centre. Participants are randomly allocated (1:1) to receive the specific multinutrient (Souvenaid) or an isocaloric, same tasting, placebo. Trial visits are done virtually using secure online video communication. English or Spanish speaking people aged 55–89 years from all ethnic groups and considered to have age-related cognitive decline are eligible. Neuropyschological tests are done at baseline and after 6 months of intervention. Participants are contacted monthly by telephone to monitor safety, assess motivation and promote compliance. The primary outcome is feasibility determined by assessing recruitment rate, recruitment time, adherence rate and retention rate. A comprehensive set of neuropyschological measures will provide a broad assessment of cognitive function, including verbal memory, processing speed, and attention and executive function. Self-reported questionnaires are used to assess quality of life. This pilot trial will provide data to guide inform selection of participants and outcome measures in future studies in age-related cognitive decline.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Jpad-Journal of Prevention of Alzheimers Disease
CLINICAL NEUROLOGY-
自引率
7.80%
发文量
85
期刊介绍:
The JPAD « Journal of Prevention of Alzheimer’Disease » will publish reviews, original research articles and short reports to improve our knowledge in the field of Alzheimer prevention including : neurosciences, biomarkers, imaging, epidemiology, public health, physical cognitive exercise, nutrition, risk and protective factors, drug development, trials design, and heath economic outcomes.
JPAD will publish also the meeting abstracts from Clinical Trial on Alzheimer Disease (CTAD) and will be distributed both in paper and online version worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


