用一品红提取物指示剂演示:寒假在家酸碱化学
IF 2.5
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
一品红(Euphorbia pulcherrima)含有可溶于水的花青素,并因pH值变化而呈现颜色变化。这一特性使一品红提取物成为pH值演示的有用酸碱指示剂。本实验与普通家居材料相结合,可作为一种低成本的教学工具,用于酸碱化学和有机化学的教学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
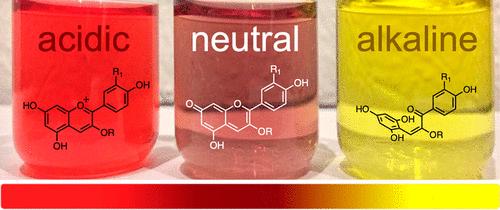
Demonstrations with Poinsettia Extract Indicator: Acid–Base Chemistry at Home During Winter Holidays
Poinsettia (Euphorbia pulcherrima) contains anthocyanins that dissolve in water and exhibit color changes due to changing pH. This property makes the poinsettia extract a useful acid–base indicator for pH demonstrations. When combined with common household materials, this experiment can be used as an alternative low-cost tool in teaching acid–base chemistry and organic chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


