用于串行飞秒晶体学的原生硫/氯 SAD 相位。
IF 2.2
4区 生物学
Acta Crystallographica Section D: Biological Crystallography
Pub Date : 2015-12-01
Epub Date: 2015-11-27
DOI:10.1107/S139900471501857X
引用次数: 0
摘要
串行飞秒晶体学(SFX)可以在辐射损伤最小的情况下确定晶体结构。然而,在 SFX 中对原生晶体进行相位测定并不常见。在此,我们利用波长为 1.77 Å 的硫和氯的反常信号,通过单波长反常衍射(SAD)成功地测定了原生溶菌酶的结构。这种硫SAD方法可应用于多种蛋白质,从而改进原生晶体结构的测定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
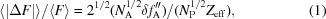

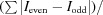
Native sulfur/chlorine SAD phasing for serial femtosecond crystallography.
Serial femtosecond crystallography (SFX) allows structures to be determined with minimal radiation damage. However, phasing native crystals in SFX is not very common. Here, the structure determination of native lysozyme from single-wavelength anomalous diffraction (SAD) by utilizing the anomalous signal of sulfur and chlorine at a wavelength of 1.77 Å is successfully demonstrated. This sulfur SAD method can be applied to a wide range of proteins, which will improve the determination of native crystal structures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
13.60%
发文量
0
审稿时长
3 months
期刊介绍:
Acta Crystallographica Section D welcomes the submission of articles covering any aspect of structural biology, with a particular emphasis on the structures of biological macromolecules or the methods used to determine them.
Reports on new structures of biological importance may address the smallest macromolecules to the largest complex molecular machines. These structures may have been determined using any structural biology technique including crystallography, NMR, cryoEM and/or other techniques. The key criterion is that such articles must present significant new insights into biological, chemical or medical sciences. The inclusion of complementary data that support the conclusions drawn from the structural studies (such as binding studies, mass spectrometry, enzyme assays, or analysis of mutants or other modified forms of biological macromolecule) is encouraged.
Methods articles may include new approaches to any aspect of biological structure determination or structure analysis but will only be accepted where they focus on new methods that are demonstrated to be of general applicability and importance to structural biology. Articles describing particularly difficult problems in structural biology are also welcomed, if the analysis would provide useful insights to others facing similar problems.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


