Wei Wang, Xiaohui Zhu, Xuecong Zhang, Chengyong Lei, Zhicheng Zeng, Xiaoliang Lan, Wenzhi Cui, Feifei Wang, Shaowan Xu, Juan Zhou, Xuehui Wu, Haijun Deng, Xia Li, Jianbing Fan, Yanqing Ding, Zhongxi Huang, Li Liang
下载PDF
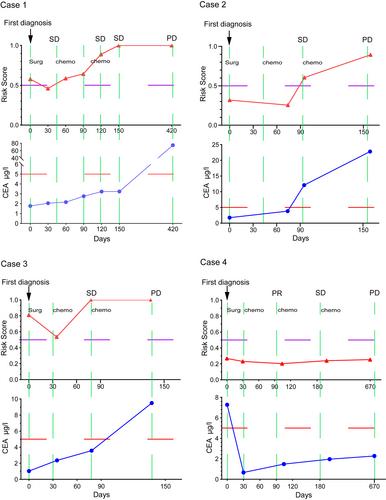
{"title":"基于血浆无细胞DNA中五种甲基化生物标志物的III期结直肠癌复发风险评估","authors":"Wei Wang, Xiaohui Zhu, Xuecong Zhang, Chengyong Lei, Zhicheng Zeng, Xiaoliang Lan, Wenzhi Cui, Feifei Wang, Shaowan Xu, Juan Zhou, Xuehui Wu, Haijun Deng, Xia Li, Jianbing Fan, Yanqing Ding, Zhongxi Huang, Li Liang","doi":"10.1002/path.6047","DOIUrl":null,"url":null,"abstract":"<p>For stage III colorectal cancer (CRC) patients with a high risk of recurrence, intensified adjuvant chemotherapy can improve overall survival. We aimed to develop a circulating tumor DNA (ctDNA) methylation marker model for predicting the relapse risk of stage III CRC patients. Differentially methylated markers identified between 53 normal mucosa samples and 165 CRC tissue samples, as well as between plasma samples from 75 stage I/II (early-stage) CRC patients and 55 stage IV (late-stage) CRC patients, were analyzed using Student's <i>t</i>-tests. The overlapping methylation markers shared by plasma and tissue samples were used to establish a methylation marker model to evaluate the tumor burden in the peripheral blood of CRC patients using the random forest method. This model was verified in the validation cohort (<i>n</i> = 44) and then applied to predict recurrence risk in 50 stage III CRC patients and monitor the clinical disease course in serial samples from four CRC patients. We built a five-marker-based ctDNA methylation model that had high sensitivity (84.21%) and specificity (84%) in identifying late-stage CRC in a validation cohort containing 24 stage I/II CRC patients and 20 stage IV CRC patients. The model achieved high sensitivity (87.5%) and specificity (94.12%) in predicting tumor relapse in an independent cohort of 50 stage III CRC patients and could be an independent recurrence risk factor for stage III patients [Hazard ratio (HR), 60.4; 95% confidence interval (CI): 7.68–397; <i>p</i> = 9.73e−5]. Analysis of serial blood samples of CRC showed that the model could monitor disease relapse earlier than imaging examination and serum carcinoembryonic antigen (CEA) and so may provide an opportunity for the early adjustment of therapeutic strategies. Moreover, the model could potentially monitor the clinical course and treatment response dynamically. © 2022 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"259 4","pages":"376-387"},"PeriodicalIF":5.6000,"publicationDate":"2022-12-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6047","citationCount":"1","resultStr":"{\"title\":\"Recurrence risk assessment for stage III colorectal cancer based on five methylation biomarkers in plasma cell-free DNA\",\"authors\":\"Wei Wang, Xiaohui Zhu, Xuecong Zhang, Chengyong Lei, Zhicheng Zeng, Xiaoliang Lan, Wenzhi Cui, Feifei Wang, Shaowan Xu, Juan Zhou, Xuehui Wu, Haijun Deng, Xia Li, Jianbing Fan, Yanqing Ding, Zhongxi Huang, Li Liang\",\"doi\":\"10.1002/path.6047\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>For stage III colorectal cancer (CRC) patients with a high risk of recurrence, intensified adjuvant chemotherapy can improve overall survival. We aimed to develop a circulating tumor DNA (ctDNA) methylation marker model for predicting the relapse risk of stage III CRC patients. Differentially methylated markers identified between 53 normal mucosa samples and 165 CRC tissue samples, as well as between plasma samples from 75 stage I/II (early-stage) CRC patients and 55 stage IV (late-stage) CRC patients, were analyzed using Student's <i>t</i>-tests. The overlapping methylation markers shared by plasma and tissue samples were used to establish a methylation marker model to evaluate the tumor burden in the peripheral blood of CRC patients using the random forest method. This model was verified in the validation cohort (<i>n</i> = 44) and then applied to predict recurrence risk in 50 stage III CRC patients and monitor the clinical disease course in serial samples from four CRC patients. We built a five-marker-based ctDNA methylation model that had high sensitivity (84.21%) and specificity (84%) in identifying late-stage CRC in a validation cohort containing 24 stage I/II CRC patients and 20 stage IV CRC patients. The model achieved high sensitivity (87.5%) and specificity (94.12%) in predicting tumor relapse in an independent cohort of 50 stage III CRC patients and could be an independent recurrence risk factor for stage III patients [Hazard ratio (HR), 60.4; 95% confidence interval (CI): 7.68–397; <i>p</i> = 9.73e−5]. Analysis of serial blood samples of CRC showed that the model could monitor disease relapse earlier than imaging examination and serum carcinoembryonic antigen (CEA) and so may provide an opportunity for the early adjustment of therapeutic strategies. Moreover, the model could potentially monitor the clinical course and treatment response dynamically. © 2022 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"259 4\",\"pages\":\"376-387\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2022-12-27\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6047\",\"citationCount\":\"1\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6047\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6047","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 1
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


