更有效的4-二甲氨基吡啶类似物的开发
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 39
摘要
介绍了两种新型亲核催化剂——双环二氨基吡啶3、4和三环三氨基吡啶5、6的合成。预测这些催化剂优于DMAP甚至2,迄今为止报道的最好的酯化催化剂。DMAP、PPY和2?比较了6种催化剂催化叔醇酯化反应的效果。正如预测的那样,5和6比DMAP有效约6倍,略好于2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
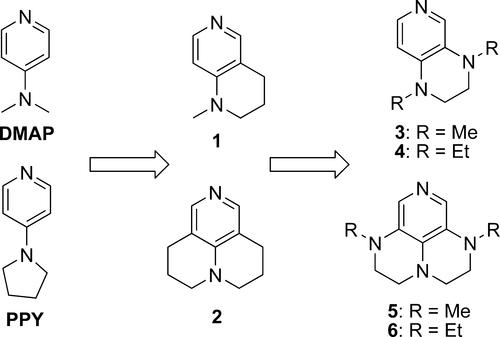
Development of More Potent 4-Dimethylaminopyridine Analogues
The syntheses of bicyclic diaminopyridines 3 and 4 and tricyclic triaminopyridines 5 and 6, two novel series of nucleophilic catalysts, are described. Arguments are made for predicting the superiority of these catalysts over DMAP and even 2, the best esterification catalyst reported to date. The efficiencies of DMAP, PPY, and 2?6 in catalyzing the esterification of tertiary alcohols were compared. As predicted, 5 and 6 were about 6-fold more effective than DMAP and slightly better than 2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


