无水四丁基氟化铵
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 283
摘要
以六氟苯为原料,采用氰化四丁基铵在低温下亲核芳烃取代制得四丁基氟化铵(TBAF)。在这个合成过程中,外来的水被生成的六正苯基清除,它在基本条件下很容易加水。与预期相反,在无水条件下,TBAF在极性非质子溶剂中对霍夫曼消去是稳定的。添加羟基溶剂可以催化TBAF的分解和催化与DMSO的质子交换。简述了该盐的合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
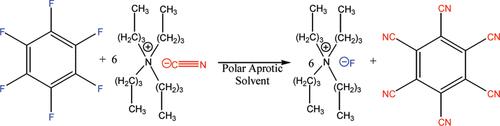
Anhydrous Tetrabutylammonium Fluoride
Tetrabutylammonium fluoride (TBAF) is prepared at low temperature by nucleophilic aromatic substitution of hexafluorobenzene with tetrabutylammonium cyanide. Adventitious water is scavenged during this synthesis by the generated hexacyanobenzene, which readily adds water under basic conditions. Contrary to expectations, TBAF is stable to Hofmann elimination in polar aprotic solvents under anhydrous conditions. Added hydroxylic solvents are shown to catalyze the decomposition of TBAF and to catalyze proton exchange with DMSO. The synthetic utility of this salt is described briefly.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


