通过本地生产扩大CAR T细胞疗法的使用范围。
IF 33.1
1区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
嵌合抗原受体(CAR)T细胞正在改变血液系统恶性肿瘤的治疗格局。迄今为止,美国食品药品监督管理局(FDA)批准的所有六种CAR T细胞产品都是自体的,并集中生产。随着获批产品和适应症的数量持续增长,迫切需要新的策略来提高细胞制造能力,以确保患者能够获得。在护理点或其他当地制造场所进行分布式制造将大大有助于满足不断增长的需求。为了确保成功实施,必须利用新技术在地理位置分散的设施中实现统一的产品质量。这包括使用自动化细胞生产系统、在线传感器和过程模拟,以增强质量控制和高效的供应链管理。全面了解CAR T细胞的关键质量属性将有助于更好地定义可广泛获得的释放标准。为了补充国家监管机构的监督,我们建议扩大认证机构的作用。此外,可能需要修订监管标准,以适应分布式制造模型的独特特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
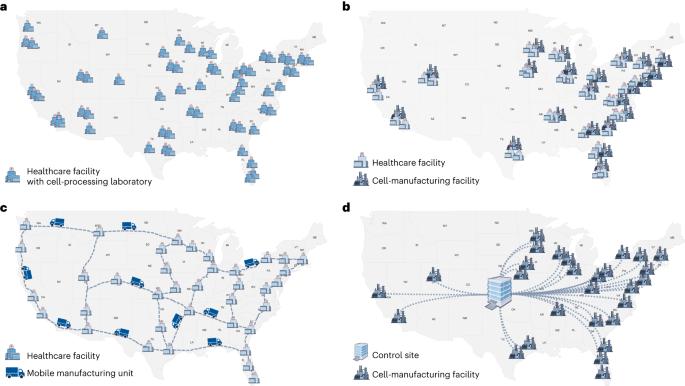
Expanding access to CAR T cell therapies through local manufacturing
Chimeric antigen receptor (CAR) T cells are changing the therapeutic landscape for hematological malignancies. To date, all six CAR T cell products approved by the US Food and Drug Administration (FDA) are autologous and centrally manufactured. As the numbers of approved products and indications continue to grow, new strategies to increase cell-manufacturing capacity are urgently needed to ensure patient access. Distributed manufacturing at the point of care or at other local manufacturing sites would go a long way toward meeting the rising demand. To ensure successful implementation, it is imperative to harness novel technologies to achieve uniform product quality across geographically dispersed facilities. This includes the use of automated cell-production systems, in-line sensors and process simulation for enhanced quality control and efficient supply chain management. A comprehensive effort to understand the critical quality attributes of CAR T cells would enable better definition of widely attainable release criteria. To supplement oversight by national regulatory agencies, we recommend expansion of the role of accreditation bodies. Moreover, regulatory standards may need to be amended to accommodate the unique characteristics of distributed manufacturing models. Shortages of CAR T cells should be alleviated by distributed manufacturing, according to Elsallab and Maus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature biotechnology
工程技术-生物工程与应用微生物
CiteScore
63.00
自引率
1.70%
发文量
382
审稿时长
3 months
期刊介绍:
Nature Biotechnology is a monthly journal that focuses on the science and business of biotechnology. It covers a wide range of topics including technology/methodology advancements in the biological, biomedical, agricultural, and environmental sciences. The journal also explores the commercial, political, ethical, legal, and societal aspects of this research.
The journal serves researchers by providing peer-reviewed research papers in the field of biotechnology. It also serves the business community by delivering news about research developments. This approach ensures that both the scientific and business communities are well-informed and able to stay up-to-date on the latest advancements and opportunities in the field.
Some key areas of interest in which the journal actively seeks research papers include molecular engineering of nucleic acids and proteins, molecular therapy, large-scale biology, computational biology, regenerative medicine, imaging technology, analytical biotechnology, applied immunology, food and agricultural biotechnology, and environmental biotechnology.
In summary, Nature Biotechnology is a comprehensive journal that covers both the scientific and business aspects of biotechnology. It strives to provide researchers with valuable research papers and news while also delivering important scientific advancements to the business community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


