在富含厌氧乙酸盐的氧化群落中,不同的电子载体驱动共营养相互作用。
IF 10.8
1区 环境科学与生态学
Q1 ECOLOGY
引用次数: 0
摘要
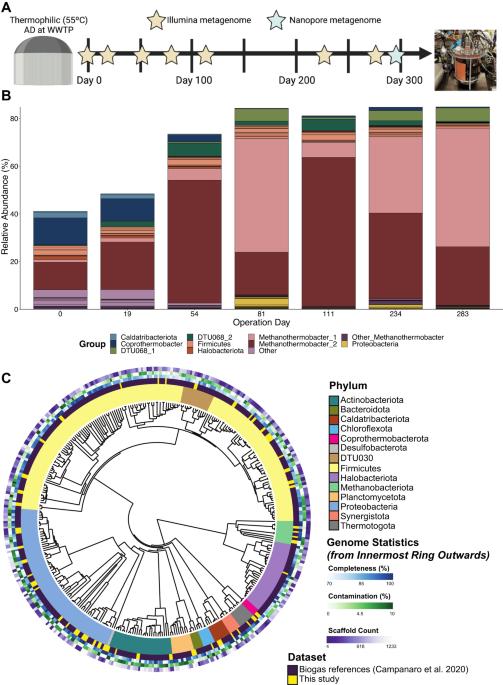
在许多缺氧环境中,共养乙酸盐氧化(SAO)是通过SAO细菌(SAOB)和产甲烷古菌之间的专性交叉喂养相互作用介导乙酸盐转化为甲烷的关键途径。SAO途径在工程环境中尤其重要,例如在高温和/或高氨条件下运行的厌氧消化(AD)系统。尽管SAOB对AD过程的稳定性具有广泛的重要性,但由于通常生物量产量低和对分离的抗性,人们对其原位生理学知之甚少。在这里,我们对嗜热菌(55 °C)使用乙酸盐作为唯一碳源的市政AD系统的SAO群落。超过80%的富集生物反应器宏基因组属于一个三成员联盟,包括一个隶属于DTU068的乙酸盐氧化细菌,编码二氧化碳、氢气和甲酸盐的产生,以及两个隶属于甲烷另一细菌-a的产甲烷古菌。稳定同位素探测与化生基因组学相结合,以量化乙酸盐转化过程中进入每个群落成员的碳通量,并为代谢重建和基因组规模建模提供信息。这项研究表明,两种甲烷另一细菌-A在其首选的电子供体方面存在差异,其中一种具有在甲酸盐上生长的能力,另一种仅消耗氢气。热力学分析表明,消耗甲酸盐的产甲烷菌的存在拓宽了SAO生产ATP的有利环境条件。总的来说,这些结果突显了SAO过程中电子分配的灵活性如何可能通过热力学驱动的互惠关系来控制群落结构和适应性,为产甲烷生态系统中这一关键官能团的代谢基础提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Diverse electron carriers drive syntrophic interactions in an enriched anaerobic acetate-oxidizing consortium
In many anoxic environments, syntrophic acetate oxidation (SAO) is a key pathway mediating the conversion of acetate into methane through obligate cross-feeding interactions between SAO bacteria (SAOB) and methanogenic archaea. The SAO pathway is particularly important in engineered environments such as anaerobic digestion (AD) systems operating at thermophilic temperatures and/or with high ammonia. Despite the widespread importance of SAOB to the stability of the AD process, little is known about their in situ physiologies due to typically low biomass yields and resistance to isolation. Here, we performed a long-term (300-day) continuous enrichment of a thermophilic (55 °C) SAO community from a municipal AD system using acetate as the sole carbon source. Over 80% of the enriched bioreactor metagenome belonged to a three-member consortium, including an acetate-oxidizing bacterium affiliated with DTU068 encoding for carbon dioxide, hydrogen, and formate production, along with two methanogenic archaea affiliated with Methanothermobacter_A. Stable isotope probing was coupled with metaproteogenomics to quantify carbon flux into each community member during acetate conversion and inform metabolic reconstruction and genome-scale modeling. This effort revealed that the two Methanothermobacter_A species differed in their preferred electron donors, with one possessing the ability to grow on formate and the other only consuming hydrogen. A thermodynamic analysis suggested that the presence of the formate-consuming methanogen broadened the environmental conditions where ATP production from SAO was favorable. Collectively, these results highlight how flexibility in electron partitioning during SAO likely governs community structure and fitness through thermodynamic-driven mutualism, shedding valuable insights into the metabolic underpinnings of this key functional group within methanogenic ecosystems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ISME Journal
环境科学-生态学
CiteScore
22.10
自引率
2.70%
发文量
171
审稿时长
2.6 months
期刊介绍:
The ISME Journal covers the diverse and integrated areas of microbial ecology. We encourage contributions that represent major advances for the study of microbial ecosystems, communities, and interactions of microorganisms in the environment. Articles in The ISME Journal describe pioneering discoveries of wide appeal that enhance our understanding of functional and mechanistic relationships among microorganisms, their communities, and their habitats.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


