Lucy Collins, Emma Boehm, Catherine Luxford, Roderick Clifton-Bligh, Vivian Grill
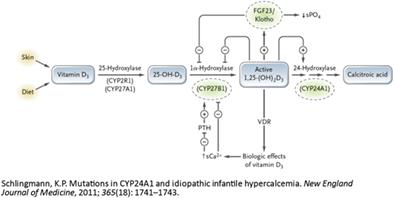
{"title":"CYP24A1功能缺失突变引起的维生素D过多:两个兄弟姐妹高钙血症的异常原因","authors":"Lucy Collins, Emma Boehm, Catherine Luxford, Roderick Clifton-Bligh, Vivian Grill","doi":"10.1002/jbm4.10788","DOIUrl":null,"url":null,"abstract":"<p>Hypervitaminosis D as a cause of hypercalcemia may be due to vitamin D intoxication, granulomatous diseases, or abnormalities of vitamin D metabolism. The <i>CYP24A1</i> gene encodes for the 24-hydroxylase enzyme, which is responsible for the catabolism of 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D). Mutations in <i>CYP24A1</i> can result in elevated 1,25(OH)2D causing parathyroid hormone (PTH)-independent hypercalcemia, hypercalciuria, nephrolithiasis, and nephrocalcinosis. We present the cases of two siblings exhibiting hypercalcemia secondary to a <i>CYP24A1</i> loss-of-function mutation. Case 1 presented initially with PTH-dependent hypercalcemia, with localization of a left upper parathyroid adenoma on parathyroid technetium sestamibi (<sup>99m</sup>Tc-MIBI) uptake study. Despite parathyroidectomy (180 mg adenoma), hypercalcemia, hypercalciuria, and low normal PTH levels persisted. A repeat parathyroid <sup>99m</sup>Tc-MIBI uptake study localized a second adenoma and a right inferior parathyroidectomy was performed (170 mg adenoma). PTH subsequently became undetectable, however hypercalcemia and hypercalciuria persisted. A new presentation of PTH-independent hypercalcemia found to be secondary to a <i>CYP24A1</i> loss-of-function mutation in his sibling, Case 2, signaled the underlying cause. Cascade testing confirmed both siblings were homozygous for the pathogenic variant c.1186C>T, p.Arg396Trp (R396W) of <i>CYP24A1</i> (NM_000782.5). In clinical practice <i>CYP24A1</i> loss-of-function mutations should be considered in patients presenting with PTH-independent hypercalcemia, hypercalciuria, and 1,25(OH)2D levels in the upper normal or elevated range. Although in our case assays of 24,25(OH)2D were not available, calculation of the 25(OH)D:24,25(OH)2D ratio can assist in the diagnostic process. Possible treatments to manage the risk of hypercalcemia in patients with a <i>CYP24A1</i> loss-of-function mutation include avoidance of vitamin D oversupplementation and excessive sun exposure. Hydration and bisphosphonate therapy can be useful in managing the hypercalcemia. Although not utilized in our cases, treatment with ketoconazole, fluconazole, and rifampicin have been described as potential therapeutic options. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.</p>","PeriodicalId":14611,"journal":{"name":"JBMR Plus","volume":"7 9","pages":""},"PeriodicalIF":3.4000,"publicationDate":"2023-08-08","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10788","citationCount":"0","resultStr":"{\"title\":\"Hypervitaminosis D Secondary to a CYP24A1 Loss-of-Function Mutation: An Unusual Cause of Hypercalcemia in Two Siblings\",\"authors\":\"Lucy Collins, Emma Boehm, Catherine Luxford, Roderick Clifton-Bligh, Vivian Grill\",\"doi\":\"10.1002/jbm4.10788\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Hypervitaminosis D as a cause of hypercalcemia may be due to vitamin D intoxication, granulomatous diseases, or abnormalities of vitamin D metabolism. The <i>CYP24A1</i> gene encodes for the 24-hydroxylase enzyme, which is responsible for the catabolism of 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D). Mutations in <i>CYP24A1</i> can result in elevated 1,25(OH)2D causing parathyroid hormone (PTH)-independent hypercalcemia, hypercalciuria, nephrolithiasis, and nephrocalcinosis. We present the cases of two siblings exhibiting hypercalcemia secondary to a <i>CYP24A1</i> loss-of-function mutation. Case 1 presented initially with PTH-dependent hypercalcemia, with localization of a left upper parathyroid adenoma on parathyroid technetium sestamibi (<sup>99m</sup>Tc-MIBI) uptake study. Despite parathyroidectomy (180 mg adenoma), hypercalcemia, hypercalciuria, and low normal PTH levels persisted. A repeat parathyroid <sup>99m</sup>Tc-MIBI uptake study localized a second adenoma and a right inferior parathyroidectomy was performed (170 mg adenoma). PTH subsequently became undetectable, however hypercalcemia and hypercalciuria persisted. A new presentation of PTH-independent hypercalcemia found to be secondary to a <i>CYP24A1</i> loss-of-function mutation in his sibling, Case 2, signaled the underlying cause. Cascade testing confirmed both siblings were homozygous for the pathogenic variant c.1186C>T, p.Arg396Trp (R396W) of <i>CYP24A1</i> (NM_000782.5). In clinical practice <i>CYP24A1</i> loss-of-function mutations should be considered in patients presenting with PTH-independent hypercalcemia, hypercalciuria, and 1,25(OH)2D levels in the upper normal or elevated range. Although in our case assays of 24,25(OH)2D were not available, calculation of the 25(OH)D:24,25(OH)2D ratio can assist in the diagnostic process. Possible treatments to manage the risk of hypercalcemia in patients with a <i>CYP24A1</i> loss-of-function mutation include avoidance of vitamin D oversupplementation and excessive sun exposure. Hydration and bisphosphonate therapy can be useful in managing the hypercalcemia. Although not utilized in our cases, treatment with ketoconazole, fluconazole, and rifampicin have been described as potential therapeutic options. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.</p>\",\"PeriodicalId\":14611,\"journal\":{\"name\":\"JBMR Plus\",\"volume\":\"7 9\",\"pages\":\"\"},\"PeriodicalIF\":3.4000,\"publicationDate\":\"2023-08-08\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10788\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"JBMR Plus\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10788\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q2\",\"JCRName\":\"ENDOCRINOLOGY & METABOLISM\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"JBMR Plus","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10788","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


