发布信息TOC
Q3 Biochemistry, Genetics and Molecular Biology
引用次数: 0
摘要
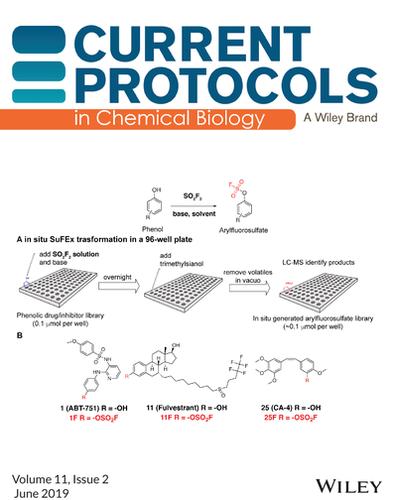
封面:Liu et al. (https://doi.org/10.1002/cpch.64), (A)在96孔板中使用饱和SO2F2溶液对苯酚进行氟磺化。(B)用结晶盐1-(氟硫酰基)-2,3-二甲基- 1h -咪唑-3-ium三氟甲烷磺酸氟磺化苯酚、仲胺和伯胺。在不同的反应条件下,分别得到芳基氟磺酸盐、磺酰氟和nh -磺酰氟(或双(氟磺酰)亚胺)。看到e64。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Issue Information TOC
Cover: In Liu et al. (https://doi.org/10.1002/cpch.64), (A) Fluorosulfurylation of phenols using saturated SO2F2 solution in a 96-well plate. (B) Fluorosulfurylation of phenols, secondary amines, and primary amines with the crystalline salt 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate. Arylfluorosulfates, sulfamoyl fluorides, and NH-sulfamoyl fluorides (or bis(fluorosulfuryl)imide, with different reaction conditions) are respectively obtained. See e64.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current protocols in chemical biology
Biochemistry, Genetics and Molecular Biology-Biophysics
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


