半胱氨酸突变的带负电荷富含脯氨酸的SAP(E)肽的有效细胞穿透
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
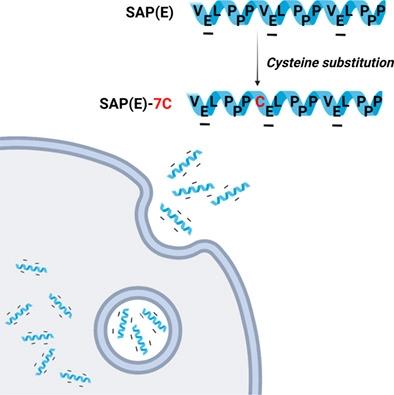
大多数细胞穿透肽(CPPs)富含带正电荷的氨基酸,但其阳离子性质可能会在实际应用中引发问题。在这项研究中,我们小心地用半胱氨酸取代了SAP(E)序列中的疏水性氨基酸,这是一种罕见的带负电荷的富含脯氨酸的CPP,以增强细胞穿透活性以及货物分子的可逆结合。大多数取代基显示出几乎可以忽略不计的细胞穿透活性,但第7缬氨酸上的半胱氨酸取代基(SAP(E)-7C)显示出比SAP(E)更能提高细胞穿透活性。当对细胞进行处理时,与带正电的TAT相比,带负电的SAP(E)-7C与酸性内涵体或溶酶体的共定位程度要低得多。SAP(E)-7C可通过与PTX的非共价络合显著增强PTX对MDA‐MB‐231细胞的作用。作为货物药物共价偶联的概念证明,巯基乙醇(一种模型药物)通过二硫键与SAP(E)-7C的半胱氨酸残基偶联,并证实了偶联物的谷胱甘肽依赖性释放。带有半胱氨酸手柄的带负电荷的SAP(E)-7C可以成为开发基于CPP的药物递送载体的有用分子模块。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effective cell penetration of negatively‐charged proline‐rich SAP(E) peptides with cysteine mutation
Most of cell penetrating peptides (CPPs) are rich in positively‐charged amino acids but the cationic property may provoke possible problems in practical applications. In this study, we carefully substituted the hydrophobic amino acids in the SAP(E) sequence, a rare example of negatively‐charged proline‐rich CPP, with cysteine for enhancement of cell penetrating activity as well as reversible conjugation of cargo molecules. Most substituents showed almost negligible cell penetrating activity, but a cysteine substituent on the 7th valine (SAP(E)‐7C) showed more improved cell penetrating activity than SAP(E). When treated to cells, the negatively‐charged SAP(E)‐7C exhibited much lower degree of co‐localization with acidic endosomes or lysosomes compared to positively‐charged TAT. SAP(E)‐7C could significantly enhance the PTX efficacy on MDA‐MB‐231 cells by non‐covalent complexation with PTX. As a proof‐of‐concept for covalent conjugation of cargo drugs, mercaptoethanol, a model drug, was conjugated to the cysteine residue of SAP(E)‐7C via a disulfide bond, and the glutathione‐dependent release from the conjugate was confirmed. The negatively‐charged SAP(E)‐7C with a cysteine handle can be a useful molecular module for the development of CPP‐based drug delivery carrier.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


