用pH开关法制备热控球形肽凝胶结构
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 1
摘要
自组装纳米结构肽凝胶是一种很有前途的传感、药物递送和能量收集材料。特别令人感兴趣的是用9-芴甲氧羰基(Fmoc)修饰的短二苯基丙氨酸(FF)肽,其促进肽构建块的结合。Fmoc-FF凝胶通常形成纤维网络,虽然其他结构已经得到证明,但对凝胶化和由此产生的有序三维结构的进一步控制可能为组织工程、传感和药物释放应用提供新的可能性。在此,我们报道了Fmoc-FF凝胶的结构可调性可以通过控制水含量和温度来实现。我们进一步探索了金属纳米颗粒在凝胶形成中的结合,以实现基于Fmoc-FF-纳米颗粒杂化微球的光学传感应用。最后,荧光寿命成像显微镜揭示了寿命和带隙减少之间的相关性,支持半导体诱导的电荷转移机制,该机制也可能增加探针分子激发态的稳定性。这些观察结果可能进一步拓宽这些肽材料在生物成像和传感应用中的用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
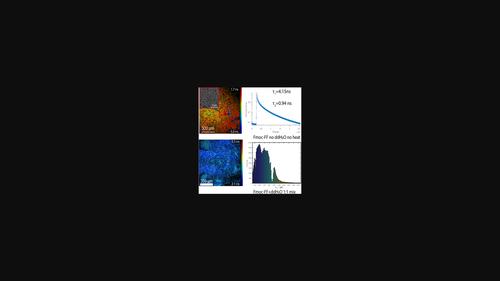
Thermally‐controlled spherical peptide gel architectures prepared using the pH switch method
Self‐assembling nanostructured peptide gels are promising materials for sensing, drug delivery, and energy harvesting. Of particular interest are short diphenylalanine (FF) peptides modified with 9‐fluorenylmethyloxycarbonyl (Fmoc), which promotes the association of the peptide building blocks. Fmoc‐FF gels generally form fibrous networks and while other structures have been demonstrated, further control of the gelation and resulting ordered three‐dimensional structures potentially offers new possibilities in tissue engineering, sensing, and drug release applications. Herein, we report that the structure tunability of Fmoc‐FF gels can be achieved by controlling the water content and the temperature. We further explore the incorporation of metal nanoparticles in the formation of the gel to enable optical sensing applications based on hybrid Fmoc‐FF‐nanoparticle microspheres. Finally, fluorescence lifetime imaging microscopy reveals a correlation between lifetime and reduced bandgap, in support of a semiconductor‐induced charge transfer mechanism that might also increase the stability of an excited state of a probe molecule. The observations potentially further widen the use of these peptide materials in bioimaging and sensing applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


