温度诱导的整合素αvβ3受体结构变化及其与RGD肽配体的相互作用
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
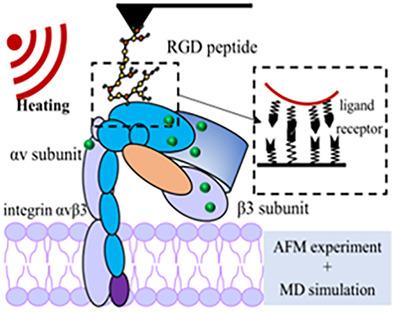
受体蛋白在高温下的结构变化是影响配体安装的纳米载体用于热疗和药物递送联合治疗的靶向能力的因素之一。本研究从理论和实验两个方面研究了整合素αvβ3受体与精氨酸-甘氨酸-天冬氨酸(RGD)肽配体在高温下的结合行为和机制。通过分子动力学模拟计算了整合素αvβ3在不同温度下的结构参数以及RGD肽与整合素αv?3在不同结合位点的相互作用力。傅里叶变换红外光谱、能量色散光谱、紫外-可见吸收光谱和原子力显微镜用于分析整合素αvβ3的结构变化并测量配体-受体相互作用。结果表明,随着温度的升高,整合素αvβ3的氢键数量减少,二级结构发生变化,表明整合素αvα3发生变性。整合素αv亚基在高温下的结构稳定性优于整合素β3亚基。RGD肽和整合素αvβ3之间的相互作用随着温度的升高而减弱,因为整合素αvα3结合位点的结构变得更加灵活,并且相应的钙离子从结合位点脱落。在310处整合素β3亚基的结合位点表现出最强的相互作用力 K,而在较高温度下在整合素αv亚基的结合位点发现,这是由于整合素αv亚基的结构变形较小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Temperature‐induced structural change of integrin αvβ3 receptor and its interaction with the RGD peptide ligand
The structural change of receptor protein at high temperatures is one of the factors affecting the targeting ability of ligand‐installed nanocarriers for combined therapy of hyperthermia and drug delivery. In this study, the binding behaviors and mechanisms of integrin αvβ3 receptor and the arginine‐glycine‐aspartic acid (RGD) peptide ligand at high temperatures were investigated both theoretically and experimentally. The structural parameters of integrin αvβ3 at different temperatures and the interaction forces between the RGD peptide and integrin αvβ3 at different binding sites were calculated by molecular dynamics simulation. Fourier transform infrared spectroscopy, energy dispersive spectroscopy, ultraviolet–visible absorption spectroscopy, and atomic force microscopy were used to analyze the structural changes of integrin αvβ3 and to measure the ligand‐receptor interaction. Results show that the number of hydrogen bonds decreased and the secondary structure of integrin αvβ3 changed with the increase in temperature, indicating the denaturation of integrin αvβ3. The structural stability of the integrin αv subunit was better than that of the integrin β3 subunit at high temperatures. The interaction between the RGD peptide and integrin αvβ3 weakened as the temperature increased because the structure of the integrin αvβ3 binding site became more flexible and the corresponding calcium ions were shed from the binding site. The strongest interaction force was exhibited at the binding site of the integrin β3 subunit at 310 K while it was found at the binding site of the integrin αv subunit at higher temperatures, owing to the smaller structure deformation of the integrin αv subunit.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


