噻唑嘧啶2-苯基腙衍生物的结构和生物学性质
IF 0.8
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 4
摘要
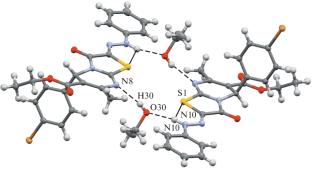
合成了一些噻唑[3,2- A]嘧啶的2-苯基腙衍生物,并对其结构和生物活性进行了研究。所得化合物的结构经1H、13C核磁共振谱和x射线衍射分析证实。得到的衍生物以C=N键的Z构型为典型的腙构型。合成的化合物对正常和肿瘤细胞系具有较低的细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure and Biological Properties of 2-Phenylhydrazone Derivatives of Thiazolopyrimidines
A number of 2-phenylhydrazone derivatives of thiazolo[3,2-a]pyrimidines has been synthesized, and their structure and biological activity have been studied. The structure of the obtained compounds has been confirmed by 1H and 13C NMR spectroscopy and X-ray diffraction analysis. It has been found that hydrazone form with Z configuration of the C=N bond is typical for the obtained derivatives. Low cytotoxicity of the synthesized compounds toward normal and tumor cell lines has been shown.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


