peptoid结构设计指南:peptoid数据库的见解
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 4
摘要
在过去的20年里,类蛋白结构研究的数量急剧增加 年。到目前为止,已经报道了超过100种蛋白胨的高分辨率结构,其通常被定义为N取代的甘氨酸低聚物。随着数据集的不断增长,我们收集了这些结构并对其序列表示进行了标准化,以便于进行结构分析。这些结构在网上被称为Peptoid数据库(database.petoids.org),它还提供了到已发布结构数据的持久链接。这篇综述分析了目前的结构集合,并发现了通过侧链在与主链氮相邻的位置的化学性质对侧链进行分组的广泛支持。在该位置具有相似化学性质的侧链组对主链的构象偏好显示出相似的影响。我们还观察到许多单体的侧链和主链构象之间的关系,这种关系以前没有引起过重大讨论:χ1和ξ二面体的值是相关的。我们概述了一种通用的设计策略,用于基于在收集的结构中看到的模式来获得特定的骨架构象。本文章由计算机程序翻译,如有差异,请以英文原文为准。
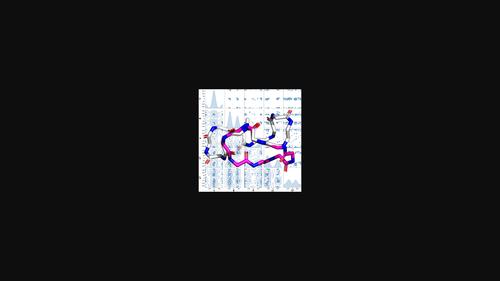
Guidelines for designing peptoid structures: Insights from the Peptoid Data Bank
The number of structural studies of peptoids has grown dramatically over the past 20 years. To date, over 100 high‐resolution structures have been reported for peptoids, which are typically defined as N‐substituted glycine oligomers. We have collected these structures and standardized their sequence representations to facilitate structural analysis as the dataset continues to grow. These structures are presented online as The Peptoid Data Bank (databank.peptoids.org), which also provides persistent links to the published structural data. This review analyzes the present collection of structures and finds extensive support for grouping side chains by their chemistry at the position adjacent to the backbone nitrogen. Groups of side chains with similar chemistry at this position show similar influences on the conformational preferences of the backbone. We also observe a relationship between the side chain and backbone conformations for many monomers that has not previously attracted significant discussion: the values of the χ1 and ϕ dihedrals are correlated. We outline a general design strategy for attaining a specific backbone conformation based on the patterns seen in the collected structures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


