先前的抗组胺药物成功地减弱了对COVID - 19疫苗的皮肤不良反应
IF 0.9
Q4 ALLERGY
引用次数: 0
摘要
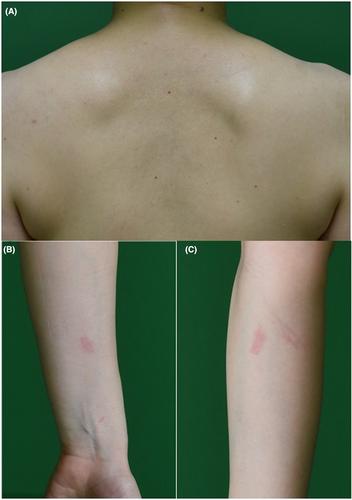
2019冠状病毒病(COVID-19)疫苗正在积极改变这场大流行的健康危机,目前对克服COVID-19-19大流行至关重要。该疫苗对感染显示出很高的疗效,并减轻了症状的严重程度。然而,这种疫苗接种与担忧有关,例如疫苗相关的不良反应,这是临床医生目前关注的问题。我们经历了两例新冠肺炎疫苗接种后的轻度皮肤不良反应,通过之前服用抗组胺药非索非那定3成功控制了这种反应 接种新冠肺炎疫苗前7天 天。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Prior antihistamine agent successfully impaired cutaneous adverse reactions to COVID-19 vaccine
The coronavirus disease 2019 (COVID-19) vaccine is positively changing the health crises of this pandemic and is currently essential to overcome the COVID-19 pandemic. The vaccine shows high efficacy against the infection and impairs the severity of symptoms. However, this vaccination is associated with concerns, such as vaccine-associated adverse reactions, which are currently highlighted issues for clinicians. We experienced two cases of mild cutaneous adverse reaction following COVID-19 vaccine administration, which was successfully controlled by prior administration of the antihistamine agent fexofenadine 3 days before COVID-19 vaccination for 7 days.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cutaneous Immunology and Allergy
Medicine-Dermatology
CiteScore
0.60
自引率
10.00%
发文量
69
审稿时长
12 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


