2,3,5,6‐四氟‐1,4‐苯二腈:具有近简并单重态和三重态的二腈
IF 1.9
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
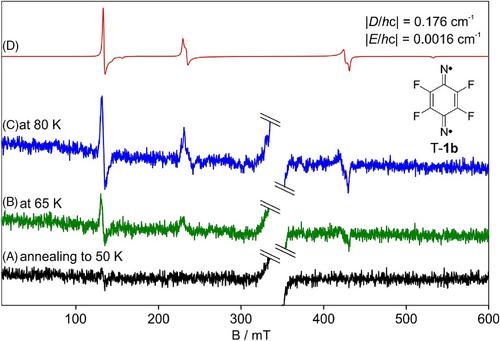
以1,4-二氮杂基2,3,5,6-四氟-1,4-苯基二硝基苯为原料,在365 nm和405 nm低温下光解合成了二硝基2,3,5,6-四氟-1,4-二硝基二硝基苯,并通过FT-IR、UV-Vis和EPR光谱对其进行了表征。二腈的电子结构最好描述为双自由基,具有开壳单重态基态和三重态,能量仅高650±5 cal/mol。λ = 254 nm辐照后,二硝基烯裂解为FCCF,烯烃裂解后裂解为FCCCN、FCCNC和FCN。本文章由计算机程序翻译,如有差异,请以英文原文为准。
2,3,5,6-Tetrafluoro-1,4-phenylenedinitrene: A dinitrene with near degenerate singlet and triplet states
The dinitrene 2,3,5,6-tetrafluoro-1,4-phenylenedinitrene was synthesized by photolysis of matrix-isolated 1,4-diazido-2,3,5,6-tetrafluorobenzene at 365 or 405 nm in high yields at cryogenic temperatures and characterized by FT-IR, UV-Vis, and EPR spectroscopy. The electronic structure of the dinitrene is described best as a quinoidal diradical with an open-shell singlet ground state and a triplet state laying only 650 ± 5 cal/mol higher in energy. Irradiation with λ = 254 nm results in fragmentation of the dinitrene into FCCF and several olefins which on prolonged photolysis fragment into FCCCN, FCCNC, and FCN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
11.10%
发文量
161
审稿时长
2.3 months
期刊介绍:
The Journal of Physical Organic Chemistry is the foremost international journal devoted to the relationship between molecular structure and chemical reactivity in organic systems. It publishes Research Articles, Reviews and Mini Reviews based on research striving to understand the principles governing chemical structures in relation to activity and transformation with physical and mathematical rigor, using results derived from experimental and computational methods. Physical Organic Chemistry is a central and fundamental field with multiple applications in fields such as molecular recognition, supramolecular chemistry, catalysis, photochemistry, biological and material sciences, nanotechnology and surface science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


