Pt-ReOx /TiO2催化剂上羧酸加氢的关键中间体
IF 1.3
Q4 ENGINEERING, CHEMICAL
引用次数: 0
摘要
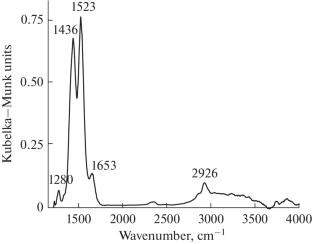
研究了吸附的乙酸形态在Pt-ReOx /TiO2催化剂上的反应性。通过原位傅立叶红外光谱在200°С下鉴定了三种吸附形式的乙酸:双齿醋酸酯和两种分子吸附形式的乙酸。发现两种形式的分子吸附乙酸的消耗速率常数(分别为0.02和0.029 s-1)与在200°С下测量的催化反应常数(0.034 s-1)的数量级接近。结果表明,这两种形式的分子吸附乙酸是Pt-ReOx /TiO2催化剂上醋酸加氢的关键中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Key Intermediates in the Hydrogenation of Carboxylic Acids on the Pt–ReOx/TiO2 Catalyst
The reactivity of adsorbed acetic acid forms on the Pt–ReOx/TiO2 catalyst has been studied. Three adsorbed acetic acid forms were identified by in situ Fourier IR spectroscopy at 200°С: bidentate acetates and two forms of molecularly adsorbed acetic acid. The consumption rate constants two forms of molecularly adsorbed acetic acid (0.02 and 0.029 s–1, respectively) were found to be close in magnitude to the catalytic reaction constant rate (0.034 s–1) measured at 200°С. It was concluded that these two forms of molecularly adsorbed acetic acid are key intermediates in acetic acid hydrogenation on the Pt–ReOx/TiO2 catalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis in Industry
ENGINEERING, CHEMICAL-
CiteScore
1.30
自引率
14.30%
发文量
21
期刊介绍:
The journal covers the following topical areas:
Analysis of specific industrial catalytic processes: Production and use of catalysts in branches of industry: chemical, petrochemical, oil-refining, pharmaceutical, organic synthesis, fuel-energetic industries, environment protection, biocatalysis; technology of industrial catalytic processes (generalization of practical experience, improvements, and modernization); technology of catalysts production, raw materials and equipment; control of catalysts quality; starting, reduction, passivation, discharge, storage of catalysts; catalytic reactors.Theoretical foundations of industrial catalysis and technologies: Research, studies, and concepts : search for and development of new catalysts and new types of supports, formation of active components, and mechanochemistry in catalysis; comprehensive studies of work-out catalysts and analysis of deactivation mechanisms; studies of the catalytic process at different scale levels (laboratory, pilot plant, industrial); kinetics of industrial and newly developed catalytic processes and development of kinetic models; nonlinear dynamics and nonlinear phenomena in catalysis: multiplicity of stationary states, stepwise changes in regimes, etc. Advances in catalysis: Catalysis and gas chemistry; catalysis and new energy technologies; biocatalysis; nanocatalysis; catalysis and new construction materials.History of the development of industrial catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


