双模EPR法研究LiCoO2的电化学循环
引用次数: 1
摘要
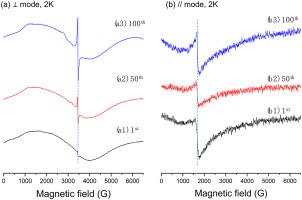
采用双模电子顺磁共振(EPR)谱分析了锂钴氧化物LiCoO2 (LCO)正极材料在锂化和锂化过程中的氧化还原机理。在循环过程中,O3-II不能完全转化回原始的O3-I相,而氧空位的产生和积累很快。我们的研究为今后更好地理解不同元素对LiCoO2掺杂的影响奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The study of electrochemical cycle for LiCoO2 by dual-mode EPR
Dual-mode electron paramagnetic resonance (EPR) spectroscopy was employed to analyze redox mechanisms in lithium cobalt oxide LiCoO2 (LCO) cathode material during delithiation and lithiation. It was found that the O3-II could not fully convert back to the pristine O3–I phase while oxygen vacancies quickly generate and accumulate during the cycling. Our study paves the way for better understanding the doping effects of different elements on LiCoO2 in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Magnetic Resonance Letters
Analytical Chemistry, Spectroscopy, Radiology and Imaging, Biochemistry, Genetics and Molecular Biology (General)
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


