在水存在下吖啶与乙基芳基-2-氧丁-3-乙酸酯同时N-和c -功能化:N-烯基吖啶酮-9-酮的合成
IF 1
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在乙基芳基-2-氧丁-3-炔酸酯和水的作用下,吖啶很容易同时发生N(1)-和С(9)-功能化,以80-84%的收率形成药理学上有前景的以前未知的N-烯基吖啶酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
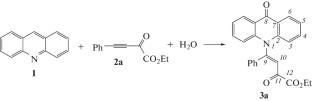
Concurrent N- and C-Functionalization of Acridine with Ethyl Aryl-2-oxobut-3-ynoates in the Presence of Water: Synthesis of N-Alkenylacridin-9-ones
Acridine has been found to undergo readily concurrent N(1)- and С(9)-functionalization under the action of ethyl aryl-2-oxobut-3-ynoates and water to form pharmacologically promising previously unknown N-alkenylacridones in 80–84% yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


