2,4,6‐三硝基甲苯资源利用反应机理研究ⅰ:N,N',N″‐三羟基异氰尿酸(THICA)催化剂在乙酸中生成2,4,6‐三硝基苯甲酸
IF 1.9
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
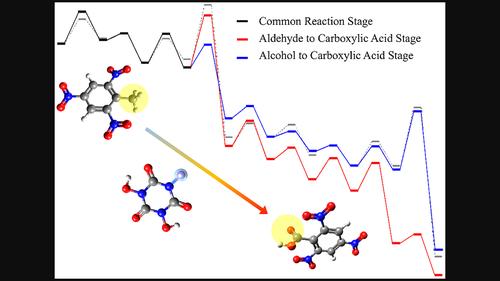
作为有机分子催化剂,N,N′,N′′-三羟基异氰尿酸可以选择性催化废2,4,6 -三硝基甲苯的甲基氧化生成2,4,6 -三硝基苯甲酸。该反应可避免无机重金属催化剂对环境的污染。本研究采用醋酸溶剂模型,在M06‐2X‐D3ZERO/6‐311G(d,p)水平上设计并计算验证了该催化反应的四个反应阶段。这些验证包括过渡态搜索、内在反应坐标计算、反应物和生成物优化以及频率计算。23个过渡态的最终反应网络表明,经过N,N',N ' -三羟基异氰尿酸活化和共同反应后,网络分为两个阶段:醇制羧酸和醛制羧酸。虽然前一阶段释放的吉布斯自由能约为155 kcal/mol,低于后一阶段释放的177 kcal/mol,但总体反应方程表明,包含前一阶段的途径不消耗催化活性物质IM_T2,节省了再活化所需的能量,因此更为有利。此外,反应网络中的关键过渡态包括双分子取代反应和质子跳跃转移反应。对它们的相互作用区域指标和本征反应坐标结果的分析表明,它们具有很强的选择性。此外,后者的能垒和热释放分别是前者的2倍和1.3倍。综上所述,本研究阐明了两种竞争性反应途径,并确定了更具能量优势和选择性的途径,为进一步优化2,4,6 -三硝基甲苯的工业利用提供了有益的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Research on the Reaction Mechanism of 2,4,6‐Trinitrotoluene Resource Utilization I:Formation of 2,4,6‐Trinitrobenzoic Acid in Acetic Acid with N,N',N″‐Trihydroxyisocyanuric Acid (THICA) Catalyst
As an organic molecule catalyst, N,N',N"‐trihydroxyisocyanuric acid can selectively catalyze the oxidation of the methyl group of waste 2,4,6‐trinitrotoluene to generate 2,4,6‐trinitrobenzoic acid. This reaction can avoid environmental pollution by inorganic heavy metal catalysts. In this study, four reaction stages of this catalytic reaction were designed and validated computationally at the M06‐2X‐D3ZERO/6‐311G(d,p) level using the acetic acid solvent model. These validations include transition state searches, intrinsic reaction coordinate calculations, reactant and product optimizations, and frequency calculations. The final reaction network of 23 transition states shows that after N,N',N"‐trihydroxyisocyanuric acid activation and common reaction, the network bifurcates into two stages: alcohol to carboxylic acid and aldehyde to carboxylic acid. Although the former stage releases about 155 kcal/mol of Gibbs free energy, less than the 177 kcal/mol from the latter stage, the overall reaction equation shows that the pathway including former stage does not consume the catalytically active substance IM_T2, which saves the energy required for reactivation and is thus more favorable. Furthermore, the key transition states in the reaction network include bimolecular substitution reactions and proton hopping transfer reactions. Analyses of their interaction region indicators and intrinsic reaction coordinate results demonstrate strong selectivity. Additionally, the energy barriers and heat releases of the latter are twice and 1.3 times greater than those of the former, respectively. In summary, this study elucidated two competitive reaction pathways and identified the more energetically favorable and selective pathway, it provides useful insights for further optimization of industrial utilization of 2,4,6‐trinitrotoluene.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
11.10%
发文量
161
审稿时长
2.3 months
期刊介绍:
The Journal of Physical Organic Chemistry is the foremost international journal devoted to the relationship between molecular structure and chemical reactivity in organic systems. It publishes Research Articles, Reviews and Mini Reviews based on research striving to understand the principles governing chemical structures in relation to activity and transformation with physical and mathematical rigor, using results derived from experimental and computational methods. Physical Organic Chemistry is a central and fundamental field with multiple applications in fields such as molecular recognition, supramolecular chemistry, catalysis, photochemistry, biological and material sciences, nanotechnology and surface science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


