侧链含多唑类肽的简易合成方法
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 1
摘要
天然金属酶通过利用多个咪唑部分来稳定金属中心。受自然界设计原理的启发,在配体中引入多种唑类化合物是构建过渡金属配合物的有效方法。在此,我们描述了一种合成后修饰类蛋白,在侧链上引入多种唑类化合物。唑(咪唑、吡唑、1,2,3-三唑和四唑)和含氯烷基的类蛋白胨之间的简单取代反应提供了多种含唑的类蛋白。由一种含氯烷基的类肽合成了10种含唑的类肽,并评估了每种唑的取代反应效率。我们已经鉴定出几种含有唑的类蛋白胨能够与Cu(II)和Fe(III)结合。我们的合成方法有助于扩大类蛋白的化学多样性,并开发用于金属识别和催化的新型类蛋白。本文章由计算机程序翻译,如有差异,请以英文原文为准。
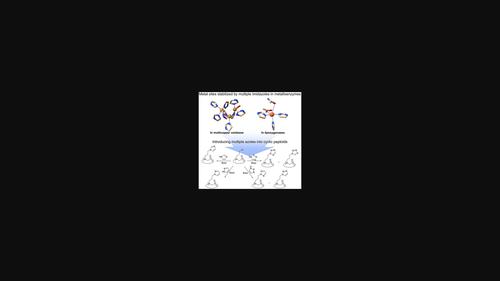
Facile synthetic method for peptoids bearing multiple azoles on side chains
Natural metalloenzymes stabilize metal centers by utilizing multiple imidazole moieties. Inspired by nature's design principles, the introduction of multiple azoles into ligands has been an effective method for constructing transition metal complexes. Herein, we describe a post‐synthetic modification of peptoids to incorporate multiple azoles on side chains. A simple substitution reaction between an azole (imidazole, pyrazole, 1,2,3‐triazole, and tetrazole) and a chloroalkyl‐containing peptoid provided access to a variety of azole‐containing peptoids. Ten azole‐containing peptoids were synthesized from a single chloroalkyl‐containing peptoid, and the efficiency of each azole for the substitution reaction was evaluated. We have identified that several of the azole‐containing peptoids are capable of binding with Cu(II) and Fe(III). Our synthetic approach can contribute to the expansion of peptoids' chemical diversity and the development of novel peptoids for metal recognition and catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


