外显子组测序允许检测癫痫患者的相关药物遗传变异
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 1
摘要
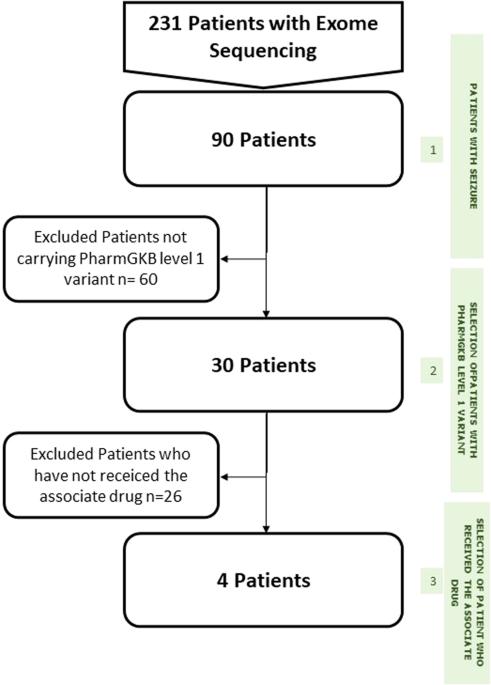
除了在孟德尔疾病诊断中鉴定因果遗传变异外,外显子组测序还能检测出许多与临床治疗潜在相关的变异。因此,可以采取临床干预措施来改善患者及其高危亲属未来的健康状况,如预测可用于预防、治疗或识别不同药物疗效和安全性的晚期遗传性疾病。为了评估此类药物基因信息的意义,我们设计了一个 "内部 "管道,以确定 31 个基因中 122 个 PharmGKB(药物基因组学知识库)变体-药物组合的状态。我们将这一方法应用于曾接受过外显子组测序(ES)分析的 90 例癫痫患者,以确定药物基因变异的频率。我们对至少携带一种相关 PharmGKB 变异的患者的药物血浆浓度和疗效进行了回顾性分析。对于 PharmGKB 1A 级变异,苯妥英处方的 CYP2C9 状态是唯一的相关信息。19名患者接受了苯妥英治疗,在接受苯妥英治疗的患者中,没有人是代谢不良者,4人是中间代谢者。在接受标准方案(10-23 毫克/千克/30 分钟的负荷剂量,然后是 5 毫克/千克/8 小时的维持剂量)治疗时,所有已确定的中等代谢者的血浆浓度都达到了中毒浓度(20 毫克/升)。在癫痫患者中,泛基因组测序可以提供常见药物基因变异的信息,这些信息可能有助于指导治疗药物监测,对于苯妥英,还可以防止因血浆浓度过高而引起的临床中毒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exome sequencing allows detection of relevant pharmacogenetic variants in epileptic patients
Beyond the identification of causal genetic variants in the diagnosis of Mendelian disorders, exome sequencing can detect numerous variants with potential relevance for clinical care. Clinical interventions can thus be conducted to improve future health outcomes for patients and their at-risk relatives, such as predicting late-onset genetic disorders accessible to prevention, treatment or identifying differential drug efficacy and safety. To evaluate the interest of such pharmacogenetic information, we designed an “in house” pipeline to determine the status of 122 PharmGKB (Pharmacogenomics Knowledgebase) variant-drug combinations in 31 genes. This pipeline was applied to a cohort of 90 epileptic patients who had previously an exome sequencing (ES) analysis, to determine the frequency of pharmacogenetic variants. We performed a retrospective analysis of drug plasma concentrations and treatment efficacy in patients bearing at least one relevant PharmGKB variant. For PharmGKB level 1A variants, CYP2C9 status for phenytoin prescription was the only relevant information. Nineteen patients were treated with phenytoin, among phenytoin-treated patients, none were poor metabolizers and four were intermediate metabolizers. While being treated with a standard protocol (10–23 mg/kg/30 min loading dose followed by 5 mg/kg/8 h maintenance dose), all identified intermediate metabolizers had toxic plasma concentrations (20 mg/L). In epileptic patients, pangenomic sequencing can provide information about common pharmacogenetic variants likely to be useful to guide therapeutic drug monitoring, and in the case of phenytoin, to prevent clinical toxicity caused by high plasma levels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


