镍催化c -氨基-1,2,4-三唑与芳香硼酸的n -芳基化反应
IF 1
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
首次研究了镍催化c -氨基-1,2,4-三唑与芳基硼酸的n -芳基化反应。建立了以氯化镍和邻菲罗啉为原料的高效催化体系,提出了制备1-取代3(5)-芳基氨基-1,2,4-三唑的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
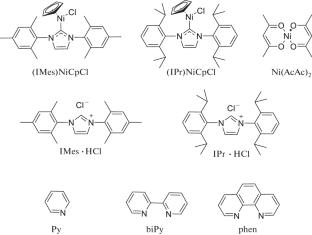
Nickel-Catalyzed N-Arylation of C-Amino-1,2,4-triazoles with Arylboronic Acids
Nickel-catalyzed N-arylation of C-amino-1,2,4-triazoles with arylboronic acids was studied for the first time. An efficient catalytic system based on nickel chloride and phenanthroline was developed and a new method for the preparation of 1-substituted 3(5)-arylamino-1,2,4-triazoles was proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


