磷酸厂循环水在鸟粪石沉淀中的应用——条件影响
IF 1.8
4区 环境科学与生态学
Q4 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
Struite是一种合适的肥料,从工业用水中电化学沉淀营养物质为循环经济提供了一个答案。铵和磷酸盐之间的摩尔比至关重要:适合沉淀的水含有比磷酸盐更多或至少相同量的铵。工业中通常不存在这种水。因此,将富含铵的工业水与富含磷的水混合,以获得合适的鸟粪石沉淀摩尔比。对参数进行了研究,以确定它们对去除率和鸟粪石产量的影响。在几种条件下,即使没有用pH控制pH,也能获得100%的鸟粪石产率 7-9。Mg:NH4:PO4(pH 9.0)。水稀释可防止镁阳极腐蚀。形成的鸟粪石具有作为回收肥料的潜力,因为通过四阶段连续浸出研究,金属的生物利用度低,营养物质的可浸出性高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
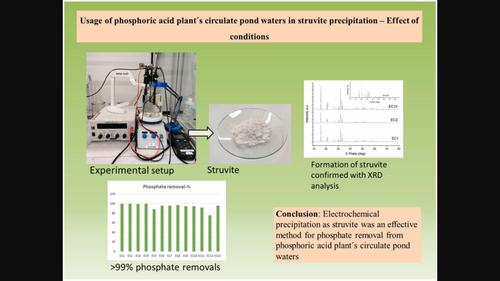
Usage of phosphoric acid plant's circulate pond waters in struvite precipitation—Effect of conditions
Struvite is a suitable fertilizer, and electrochemical precipitation of nutrients from industrial waters provides one answer to the circular economy. Molar ratio between ammonium and phosphate is crucial: Water suitable for the precipitation includes more or at least the same amount ammonium than phosphate. That kind of water typically does not exist in industry. Therefore, ammonium‐rich industrial water was mixed with phosphorus‐rich water to obtain a suitable molar ratio for struvite precipitation. Parameters were studied to determine their effect on removal‐% and struvite yield. 100% struvite yield was obtained under several conditions even without pH control with pH 7–9. The highest phosphate removal (99.7%) was occurred with the molar ratio 1.7:2:1 for Mg:NH4:PO4 (pH 9.0). Waters dilution prevents magnesium anode corrosion. Formed struvite has potential as recycled fertilizer due to low bioavailability of metals and high leachability of nutrients studied by four‐stage sequential leaching.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water and Environment Journal
环境科学-湖沼学
CiteScore
4.80
自引率
0.00%
发文量
67
审稿时长
18-36 weeks
期刊介绍:
Water and Environment Journal is an internationally recognised peer reviewed Journal for the dissemination of innovations and solutions focussed on enhancing water management best practice. Water and Environment Journal is available to over 12,000 institutions with a further 7,000 copies physically distributed to the Chartered Institution of Water and Environmental Management (CIWEM) membership, comprised of environment sector professionals based across the value chain (utilities, consultancy, technology suppliers, regulators, government and NGOs). As such, the journal provides a conduit between academics and practitioners. We therefore particularly encourage contributions focussed at the interface between academia and industry, which deliver industrially impactful applied research underpinned by scientific evidence. We are keen to attract papers on a broad range of subjects including:
-Water and wastewater treatment for agricultural, municipal and industrial applications
-Sludge treatment including processing, storage and management
-Water recycling
-Urban and stormwater management
-Integrated water management strategies
-Water infrastructure and distribution
-Climate change mitigation including management of impacts on agriculture, urban areas and infrastructure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


