区域边缘Truce–Smiles重排:KN(SiMe3)2†促进芳基吲哚合成的策略
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
公开了通过环化和区域控制的Truce–Smiles(T–S)重排化学和区域选择性合成2-苄基和2,3-二取代吲哚。由KN(SiMe3)2介导的2-氨基二苯乙炔的级联5-内-二烯环化之后是区域控制的T–S反应。该系统提供了T–S区域选择性的第一个例子,并由K+上的配体控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
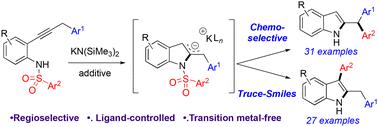
A regiodivergent Truce–Smiles rearrangement: a strategy for the synthesis of arylated indoles promoted by KN(SiMe3)2†
A chemo- and regioselective synthesis of 2-benzhydryl and 2,3-disubstituted indoles via cyclization and regiocontrolled Truce–Smiles (T–S) rearrangement is disclosed. A cascade 5-endo-dig cyclization of 2-amino diphenylacetylenes mediated by KN(SiMe3)2 is followed by a regiocontrolled T–S reaction. This system provides the first example of T–S regioselectivity and is controlled by ligands on K+.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


