BTK抑制剂BIIB068的多图谱合成
IF 2.5
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文介绍了一种相对可行、经济、安全的多流程合成高效可逆BTK抑制剂BIIB068的方法。采用简单的亲核取代反应和氰基的绿色还原,经济地构建了2-氨基-4取代的嘧啶,突显了这一新工艺。所开发的方法提供了32%的总产率,HPLC纯度为99%。BIIB068的结构通过MS、IR、1H-NMR和13C-NMR得到了证实,一些中间体的结构也通过MS和1H-NMR得到了证实本文章由计算机程序翻译,如有差异,请以英文原文为准。
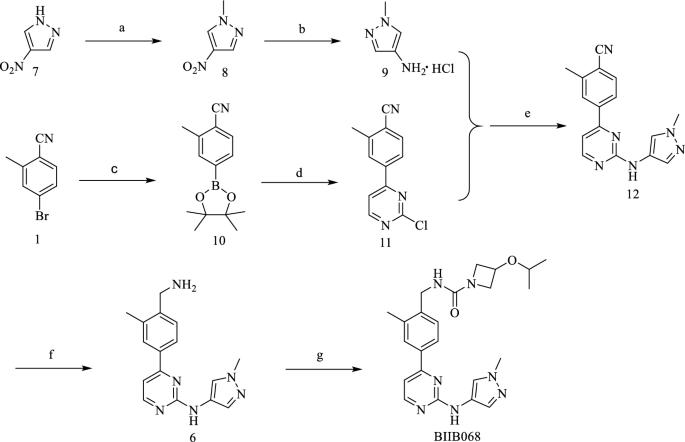
Multigram‑scale synthesis of BTK inhibitor BIIB068
This paper describes the development of a relatively feasible, economic, and safe method for the multigram-scale synthesis of BIIB068, an efficient reversible BTK inhibitor. The new process is highlighted by an economical construction of a 2-amino-4 substituted pyrimidine employing a simple nucleophilic substitution reaction and a green reduction of the cyano group. The developed process provided an overall yield of 32%, with an HPLC purity of 99%. The structure of BIIB068 was confirmed by MS, IR, 1H-NMR, and 13C-NMR, and the structures of some intermediates were confirmed by MS and 1H-NMR.
Graphical abstract
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Papers
化学-化学综合
CiteScore
3.90
自引率
4.50%
发文量
590
审稿时长
2.5 months
期刊介绍:
Chemical Papers is a peer-reviewed, international journal devoted to basic and applied chemical research. It has a broad scope covering the chemical sciences, but favors interdisciplinary research and studies that bring chemistry together with other disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


