深空生物活性化合物和抗生素发现的挑战与机遇
摘要
探索任务或殖民化的长期太空居住将对居住在重力变化的封闭栖息地的宇航员造成重大健康风险,一次居住数年,没有来自地球的补给[Taylor和Sommer(Int J Antimicrob Agents 26:183-1872005)]。有一天,火星、深空、木星和土星卫星上的永久基地可能会建立起来,使太空殖民者远离陆地物质或医疗援助。微重力、低氧水平和辐射暴露大大增加了细胞氧化应激和线粒体功能障碍、骨骼和肌肉损失、癌症风险、中枢神经系统功能障碍、,以及我们基于地球的进化谱系尚未为人类做好准备的一系列其他问题[Afshinnekoo等人(Cell 183:1162–11842020);Patel等人(NPJ Microgravity 6:32020)]。所有这些问题,加上潜在的营养不良和与有限数量的人禁闭的心理压力[Oluwafemi等人(Life Sci Space Res 28:26–312021)],以及无法接触自然世界,将不可避免地导致免疫功能下降,使宇航员更容易受到次级病原体的感染,而次级病原体通常作为人类共生体发挥作用(更不用说可以感染健康人的初级病原体)。尽管在地球上,包括抗生素在内的许多药物的保质期比以前想象的要长得多,但这些药物在太空的高辐射环境中降解得更快[Du等人(AAPS J 13:299–3082011);Blue等人(NPJ Microgravity 5:142019)]。长期执行任务的宇航员或其他星球上的殖民者可能需要能够在原地制造救生药物。特别是,如果太空栖息地出现无法用现有抗生素治疗的不可预见的抗微生物病原体,则需要开发新的抗生素。这是一个无法完全远程解决的问题,因为抗生素必须针对相关病原体进行测试。地球上使用的大多数抗生素都是从微生物或其合成变体中提取的天然产物;未来的太空殖民者需要利用他们太空栖息地的生物多样性,挖掘他们携带的任何微生物物种之间的生态关系。还必须创建或调整抗生素发现工具,以便在空间环境中使用。例如,研究太空中不同微生物相互作用的最小抑制浓度的方法,以及从国际空间站(ISS)等空间站生成常见分离物的全基因组序列数据库,用于生物合成基因簇的基因组挖掘和抗微生物耐药性的潜在机制。其他技术需求包括构建耐太空的液相色谱-质谱(LC–MS)平台,以连接到分子网络工具,以及设计抗生素化合物的发酵、提取和纯化装置,这些装置必须在计划火星或更远的任务之前很久开发,以对过程进行基准测试。从国际空间站分离的微生物的全基因组测序和完整遗传清单已经在进行中[Be等人(微生物组5:812017);Singh等人(微生物群6:2042018);Checinska-Sielaff等人(微生物区7:502019);Simpson等人(微生物资源公告10:e0751–e17212021);Simson等人。(微生物组10:1-192022)],很快就可以对国际空间站上分离的细菌和真菌进行原位测序[Stahl Rommel等人(Genes 12:1062021)]。如果没有地球的生物多样性,或者没有设备齐全的实验室在全重力下的所有工具,在太空中寻找新的生物活性化合物的过程将是困难的。然而,也有许多潜在的优势,包括蛋白质化合物在微重力下易于纯化和结晶[Borgstahl等人(完美晶体:用于中子晶体学的人锰超氧化物歧化酶的微重力毛细管反扩散结晶,2022);Martirosyan等人(npj microgravity 8:1-122022)],这种努力将引起许多科学头脑的关注,以应对太空健康问题,以及缺乏提供目前阻碍抗生素发现和生产的商业案例的必要性(Miethke等人[Nat Rev Chem 5:726–7492021)]。这一过程的第一步是创建一个框架,以解决未来太空栖息地微生物组的总体生态学问题——微重力条件下的无计划微生物群落是否形成了能够击败病原微生物的复杂生态系统?(图。
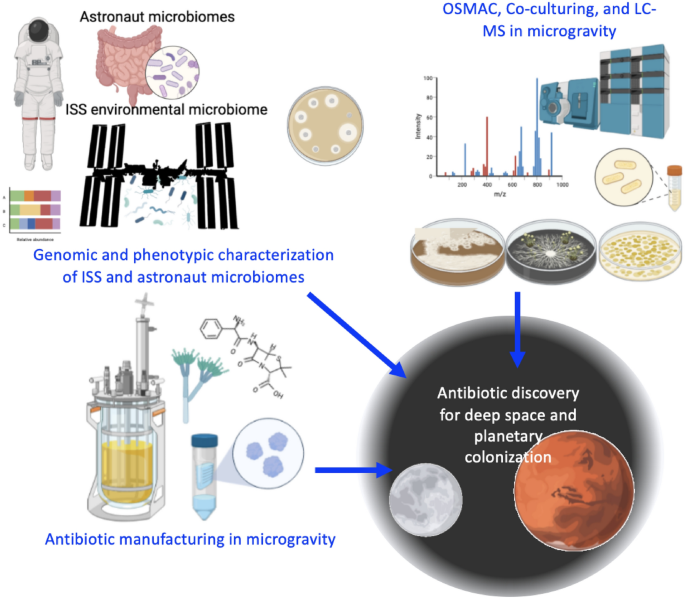
Long-term space habitation for exploratory missions or colonization will involve significant health risks to astronauts living in enclosed habitats in altered gravity, for years at a time with no resupply from Earth [Taylor and Sommer (Int J Antimicrob Agents 26:183–187, 2005)]. Permanent bases on Mars, in deep space, and on the moons of Jupiter and Saturn may one day be established, putting space colonists far out of the reach of terrestrial material or medical aid. Microgravity, low oxygen levels and radiation exposure greatly increase cellular oxidative stress and mitochondrial dysfunction, bone and muscle loss, cancer risk, central nervous system dysfunction, and a whole host of other issues for which our Earth-based evolutionary lineage has not prepared humanity [Afshinnekoo et al. (Cell 183:1162–1184, 2020); Patel et al. (NPJ Microgravity 6:33, 2020)]. All of these issues, along with potential malnutrition and the psychological stress of confinement with a limited number of individuals [Oluwafemi et al. (Life Sci Space Res 28:26–31, 2021)], and without access to the natural world, inevitably will lead to decreased immune function, making astronauts more vulnerable to infection by secondary pathogens which normally operate as human commensals (not to mention primary pathogens that can infect healthy individuals). Although on Earth the shelf life of many medications including antibiotics is much longer than previously thought, these medications degrade much more rapidly in the high-radiation environment of space [Du et al. (AAPS J 13:299–308, 2011); Blue et al. (NPJ Microgravity 5:14, 2019)]. Astronauts on long-term missions, or colonists on other planets, will likely need to be able to manufacture life-saving drugs in situ. In particular, if unforeseen antimicrobial-resistant pathogens emerge in space habitats which are not treatable with current antibiotics, new antibiotics will need to be developed. This is one problem that cannot be solved entirely remotely, as the antibiotics must be tested against the pathogen in question. Most antibiotics used on Earth are natural products derived from microbes, or synthetic variants thereof; future space colonists will need to make use of the biodiversity of their space habitats and mine the ecological relationships between whatever microbial species they bring with them. Tools for antibiotic discovery must also be created or adapted for use in the space environment. Examples are methods for studying minimum inhibitory concentrations for different microbial interactions in space, and producing databases of the whole genome sequences of common isolates from space stations such as the International Space Station (ISS) for genome mining of biosynthetic gene clusters and potential mechanisms of antimicrobial resistance. Other technology needs are building space-hardy liquid chromatography-mass spectrometry (LC–MS) platforms to link to molecular networking tools, and designing provisions for fermentation, extraction and purification of antibiotic compounds, which must be developed long before a mission to Mars or beyond is planned in order to benchmark the processes. Whole genome sequencing and a full genetic inventory of microbes isolated from the ISS is already underway [Be et al. (Microbiome 5:81, 2017); Singh et al. (Microbiome 6:204, 2018); Checinska Sielaff et al. (Microbiome 7:50, 2019); Simpson et al. (Microbiol Resource Announc 10:e00751–e1721, 2021); Simpson et al. (Microbiol Resource Announc 10:e00751–e1721, 2021); Urbaniak et al. (Microbiome 10:1–19, 2022)], and soon isolated bacteria and fungi aboard the ISS may be sequenced in situ [Stahl-Rommel et al. (Genes 12:106, 2021)]. The process of novel bioactive compound prospecting in space will be difficult without the biodiversity of Earth, or all the many tools of a fully equipped lab in full gravity. However, there are many potential advantages as well, including the ease of purification and crystallization of protein compounds in microgravity [Borgstahl et al. (Perfect crystals: microgravity capillary counterdiffusion crystallization of human manganese superoxide dismutase for neutron crystallography, 2022); Martirosyan et al. (npj Microgravity 8:1–12, 2022)], the focus and attention of many scientific minds such an endeavor would bring in response to a space health problem, and the lack of a need to provide a business case which currently bottlenecks antibiotic discovery and production (Miethke et al. [Nat Rev Chem 5:726–749, 2021)]. A first step in this process is creating a framework for addressing the general ecology of a future space habitat microbiome—do unplanned consortia of microbes in microgravity form complex ecosystems capable of outcompeting pathogenic microbes? (Fig. 1) And further, can these microbial consortia be used for new drug discovery on space missions where resupply from Earth is not possible? Or will the biodiversity of such a habitat, mission or colony be low enough that a pre-planned selection of preserved microbes or extremely diverse environmental samples such as soils should be brought along?
Strategies for future antibiotic discovery in space.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


