来自高嗜地气杆菌 HTA426 的假想 DUF1811 家族蛋白 GK0453 的结构。
IF 0.9
4区 生物学
Acta Crystallographica Section F-structural Biology and Crystallization Communications
Pub Date : 2013-04-01
Epub Date: 2013-03-28
DOI:10.1107/S1744309113003369
引用次数: 0
摘要
通过分子置换法测定了来自高嗜酸地杆菌(Geobacillus kaustophilus)的保守假定蛋白质 GK0453 的晶体结构,分辨率为 2.2 Å。该晶体属于空间群 P4(3)2(1)2,单位晶胞参数为 a = b = 75.69,c = 64.18 Å。该结构是通过分子置换法测定的,其最终 R 因子为 22.6%(R(free) = 26.3%)。根据结构同源性,GK0453 蛋白具有两个独立的结合位点,因此它可能同时与两种蛋白质或一种蛋白质和一种核酸相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
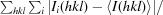
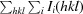
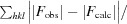
Structure of the hypothetical DUF1811-family protein GK0453 from Geobacillus kaustophilus HTA426.
The crystal structure of a conserved hypothetical protein, GK0453, from Geobacillus kaustophilus has been determined to 2.2 Å resolution. The crystal belonged to space group P4(3)2(1)2, with unit-cell parameters a = b = 75.69, c = 64.18 Å. The structure was determined by the molecular-replacement method and was refined to a final R factor of 22.6% (R(free) = 26.3%). Based on structural homology, the GK0453 protein possesses two independent binding sites and hence it may simultaneously interact with two proteins or with a protein and a nucleic acid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
自引率
0.00%
发文量
0
审稿时长
2-4 weeks
期刊介绍:
Acta Crystallographica Section F is a rapid structural biology communications journal.
Articles on any aspect of structural biology, including structures determined using high-throughput methods or from iterative studies such as those used in the pharmaceutical industry, are welcomed by the journal.
The journal offers the option of open access, and all communications benefit from unlimited free use of colour illustrations and no page charges. Authors are encouraged to submit multimedia content for publication with their articles.
Acta Cryst. F has a dedicated online tool called publBio that is designed to make the preparation and submission of articles easier for authors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


