下载PDF
{"title":"紫杉醇、异环磷酰胺和顺铂诱导化疗后同步放化疗治疗不可切除的局部晚期头颈部癌。","authors":"I Chitapanarux, E Tharavichitkul, V Lorvidhaya, P Sittitrai, T Pattarasakulchai","doi":"10.2349/biij.6.3.e23","DOIUrl":null,"url":null,"abstract":"<p><strong>Objective: </strong>Induction chemotherapy (IC) and concurrent chemoradiotherapy (CCRT) for locally advanced head and neck cancer has been studied in many clinical trials. This study was conducted to determine the response rate of IC with paclitaxel, ifosfamide, and cisplatin followed by CCRT with cisplatin for this group of patients, and the effect of the entire treatment on survival and time to disease progression.</p><p><strong>Methods: </strong>Thirty patients with advanced and unresectable head and neck cancer were treated with 2 cycles of induction paclitaxel/ ifosfamide/ cisplatin. If the primary tumor had a complete or partial response, patients were treated with 2 more cycles of IC followed by radiotherapy 70 Gy plus 3 cycles of cisplatin. For those with less than partial response or disease progression were treated according to the discretion of the physicians.</p><p><strong>Results: </strong>Ninety percent of patients had stage IV disease and 40% of them had primary tumor at maxillary sinus and nasal cavity. One patient (3%) achieved complete response (CR) and 18 patients had partial responses (PR) to IC. CCRT enhanced the response rate, resulting in a total of 3 CR (10%) and 16 PR (53%) to treatment. The median time to progression was 11.5 months. The median overall survival was 27 months. The most severe hematologic toxicity occurred during IC was grade3-4 neutropenia (40%). Grade 3-4 mucositis occurred in 68% of patients during CCRT.</p><p><strong>Conclusion: </strong>This novel combined-modality treatment program, is toxic but feasible, and can be administered for selected patients with advanced and unresectable head and neck cancer. © 2010 Biomedical Imaging and Intervention Journal. All rights reserved.</p>","PeriodicalId":89331,"journal":{"name":"Biomedical imaging and intervention journal","volume":"6 3","pages":"e23"},"PeriodicalIF":0.0000,"publicationDate":"2010-07-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.2349/biij.6.3.e23","citationCount":"0","resultStr":"{\"title\":\"Induction chemotherapy with paclitaxel, ifosfamide, and cisplatin followed by concurrent chemoradiotherapy for unresectable locally advanced head and neck cancer.\",\"authors\":\"I Chitapanarux, E Tharavichitkul, V Lorvidhaya, P Sittitrai, T Pattarasakulchai\",\"doi\":\"10.2349/biij.6.3.e23\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p><strong>Objective: </strong>Induction chemotherapy (IC) and concurrent chemoradiotherapy (CCRT) for locally advanced head and neck cancer has been studied in many clinical trials. This study was conducted to determine the response rate of IC with paclitaxel, ifosfamide, and cisplatin followed by CCRT with cisplatin for this group of patients, and the effect of the entire treatment on survival and time to disease progression.</p><p><strong>Methods: </strong>Thirty patients with advanced and unresectable head and neck cancer were treated with 2 cycles of induction paclitaxel/ ifosfamide/ cisplatin. If the primary tumor had a complete or partial response, patients were treated with 2 more cycles of IC followed by radiotherapy 70 Gy plus 3 cycles of cisplatin. For those with less than partial response or disease progression were treated according to the discretion of the physicians.</p><p><strong>Results: </strong>Ninety percent of patients had stage IV disease and 40% of them had primary tumor at maxillary sinus and nasal cavity. One patient (3%) achieved complete response (CR) and 18 patients had partial responses (PR) to IC. CCRT enhanced the response rate, resulting in a total of 3 CR (10%) and 16 PR (53%) to treatment. The median time to progression was 11.5 months. The median overall survival was 27 months. The most severe hematologic toxicity occurred during IC was grade3-4 neutropenia (40%). Grade 3-4 mucositis occurred in 68% of patients during CCRT.</p><p><strong>Conclusion: </strong>This novel combined-modality treatment program, is toxic but feasible, and can be administered for selected patients with advanced and unresectable head and neck cancer. © 2010 Biomedical Imaging and Intervention Journal. All rights reserved.</p>\",\"PeriodicalId\":89331,\"journal\":{\"name\":\"Biomedical imaging and intervention journal\",\"volume\":\"6 3\",\"pages\":\"e23\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2010-07-01\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://sci-hub-pdf.com/10.2349/biij.6.3.e23\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Biomedical imaging and intervention journal\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://doi.org/10.2349/biij.6.3.e23\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Biomedical imaging and intervention journal","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.2349/biij.6.3.e23","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Induction chemotherapy with paclitaxel, ifosfamide, and cisplatin followed by concurrent chemoradiotherapy for unresectable locally advanced head and neck cancer.
Objective: Induction chemotherapy (IC) and concurrent chemoradiotherapy (CCRT) for locally advanced head and neck cancer has been studied in many clinical trials. This study was conducted to determine the response rate of IC with paclitaxel, ifosfamide, and cisplatin followed by CCRT with cisplatin for this group of patients, and the effect of the entire treatment on survival and time to disease progression.
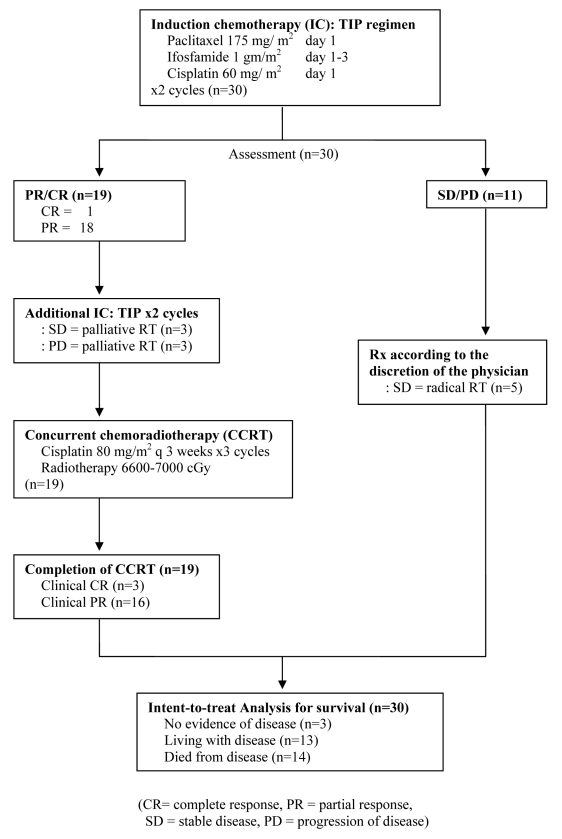
Methods: Thirty patients with advanced and unresectable head and neck cancer were treated with 2 cycles of induction paclitaxel/ ifosfamide/ cisplatin. If the primary tumor had a complete or partial response, patients were treated with 2 more cycles of IC followed by radiotherapy 70 Gy plus 3 cycles of cisplatin. For those with less than partial response or disease progression were treated according to the discretion of the physicians.
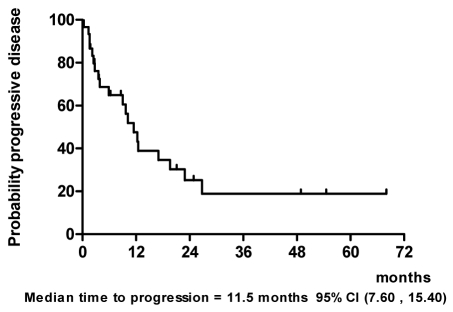
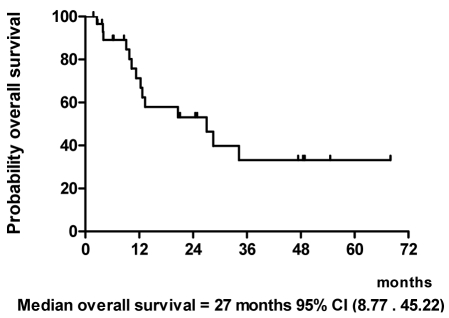
Results: Ninety percent of patients had stage IV disease and 40% of them had primary tumor at maxillary sinus and nasal cavity. One patient (3%) achieved complete response (CR) and 18 patients had partial responses (PR) to IC. CCRT enhanced the response rate, resulting in a total of 3 CR (10%) and 16 PR (53%) to treatment. The median time to progression was 11.5 months. The median overall survival was 27 months. The most severe hematologic toxicity occurred during IC was grade3-4 neutropenia (40%). Grade 3-4 mucositis occurred in 68% of patients during CCRT.
Conclusion: This novel combined-modality treatment program, is toxic but feasible, and can be administered for selected patients with advanced and unresectable head and neck cancer. © 2010 Biomedical Imaging and Intervention Journal. All rights reserved.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


